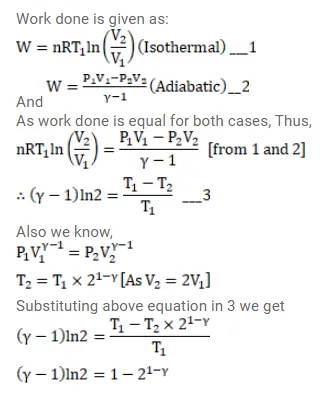Question:
Two samples $A$ and $B$ of the gas have equal volumes and pressures. The gas in sample $A$ is expanded isothermally to double its volume and the gas in $B$ is expanded adiabatically to double its volume. If the work expanded adiabatically to double its volume. If the work done by the gas is the same for the two cases, show that $\gamma_{\text {satisfies the equation }} 1-2^{1-\gamma}=(\gamma-1) \ln 2$
Solution: