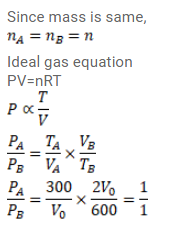Question:
Use $\mathrm{R}=8.3 \mathrm{~J} / \mathrm{mol}-\mathrm{K}$ wherever required.
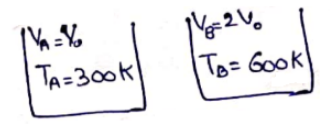
Equal of masses of air are sealed in two vessels, one of volume $V_{0}$ and the other of volume $2 V_{0}$. If the first vessel is maintained at a temperature $300 \mathrm{~K}$ and the other at $600 \mathrm{~K}$, find the ratio of the pressures in the two vessels.
Solution: