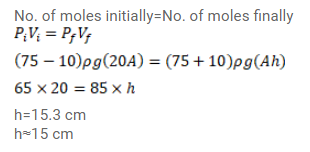Question:
Use $R=8.3 \mathrm{~J} / \mathrm{mol}-\mathrm{K}$ wherever required.
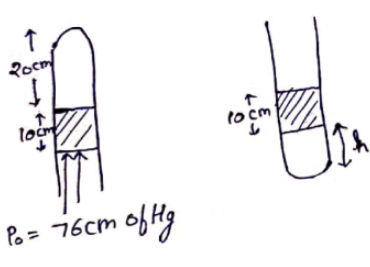
A uniform tube closed at one end, contains a pallet of mercury $10 \mathrm{~cm}$ long. When the tube is kept vertically with the closed end upward, the length of the air column trapped is $20 \mathrm{~cm}$. Find the length of the air column trapped when the tube is inverted so that the closed end goes down. Atmospheric pressure $=75 \mathrm{~cm}$ of mercury.
Solution: