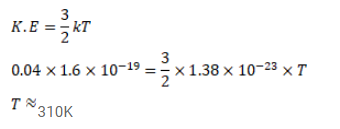Question:
Use $\mathrm{R}=8.3 \mathrm{~J} / \mathrm{mol}-\mathrm{K}$ wherever required.
The average translational kinetic energy of air molecules is $0.040 \mathrm{eV}\left(1 \mathrm{eV}=1.1 \times 10^{-19} \mathrm{~J}\right)$. Calculate the temperature of the air. Boltzmann constant $\mathrm{k}=1.38 \times 10^{-23} \mathrm{~J} / \mathrm{K}$.
Solution: