Question:
Use $\mathrm{R}=8.3 \mathrm{~J} / \mathrm{mol}-\mathrm{K}$ wherever required.
Using figure of the text, find the boiling point of methyl alcohol at $1 \mathrm{~atm}(760 \mathrm{~mm}$ of mercury) and at $0.5$ atm.
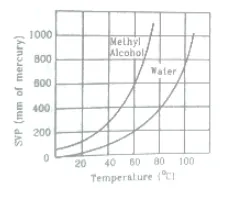
Solution:
At pressure of $760 \mathrm{~mm}$ we drop perpendicular on temperature axis, So, $\mathrm{T}=65^{\circ} \mathrm{C}$ Similarly, at $0.5$ atm, $T=48^{\circ} \mathrm{C}$
