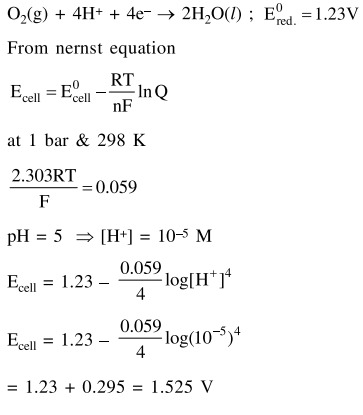
Question:
What would be the electrode potential for the given half cell reaction at $\mathrm{pH}=5$ ?_________
$2 \mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{O}_{2}+4 \mathrm{H}^{\oplus}+4 \mathrm{e}^{-} ; \mathrm{E}^{0}$ red $=1.23 \mathrm{~V}$
$\left(\mathrm{R}=8.314 \mathrm{~J} \mathrm{~mol}^{-1} \mathrm{~K}^{-1} ;\right.$ Temp $=298 \mathrm{~K} ;$ oxygen
Solution: