Question: Which among the following species has unequal bond lengths?
$\mathrm{XeF}_{4}$
$\mathrm{SiF}_{4}$
$B F_{4}^{-}$
$\mathrm{SF}_{4}$
Correct Option: , 4
Solution:
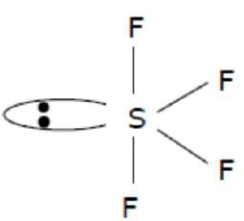
$\mathrm{Sp}^{3} \mathrm{~d}$ Hybridisation Sea-saw shape $\backslash \&$ axial bond length is more than equitorial bond length