Question:
Which of the following are isostructural pairs?
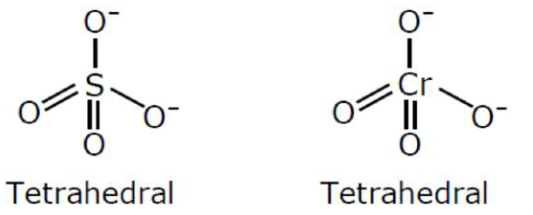
(A) $\mathrm{SO}_{4}^{2-}$ and $\mathrm{CrO}_{4}^{2-}$
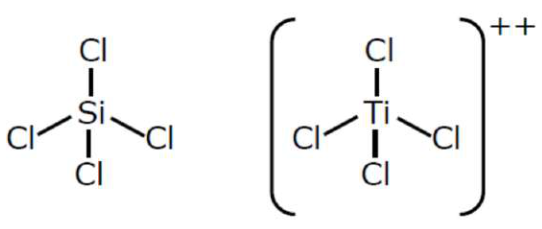
(B) $\mathrm{SiCl}_{4}$ and $\mathrm{TiCl}_{4}$
(c) $\mathrm{NH}_{3}$ and $\mathrm{NO}_{3}^{-}$
(D) $\mathrm{BCl}_{3}$ and $\mathrm{BrCl}_{3}$
Correct Option: , 2
Solution:
(a) $\mathrm{SO}_{4}^{-2}$ and $\mathrm{CrO}_{4}{ }^{2-}$ both have tetrahedral structure.
(b) $\mathrm{SiCl}_{4}$ and $\mathrm{TiCl}_{4}$ both have Tetrahedral structure also.