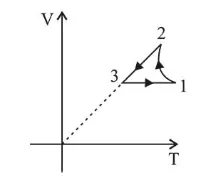
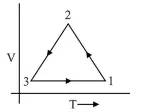
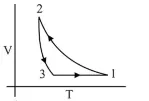
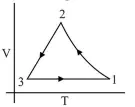
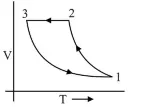
Which of the following is an equivalent cyclic process corresponding to the thermodynamic cyclic given in the figure?
Question:
Which of the following is an equivalent cyclic process corresponding to the thermodynamic cyclic given in the figure?
where, $1 \rightarrow 2$ is adiabatic.
(Graphs are schematic and are not to scale)
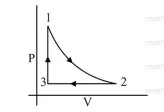
Correct Option: , 3
Solution:
(3) For process $3 \rightarrow 1$ volume is constant
$\therefore \quad$ Graph given in option (4) is wrong.
And process $1 \rightarrow 2$ is adiabatic $\therefore$ graph in option (1) is wrong
$\because \quad v=$ constant
$P \uparrow, T \uparrow$
For Process $2 \rightarrow 3$ Pressure constant i.e., $P=$ constant
$\therefore \quad V \downarrow T \downarrow$
Hence graph (3) is the correct $V-T$ graph of given $P-V$ graph