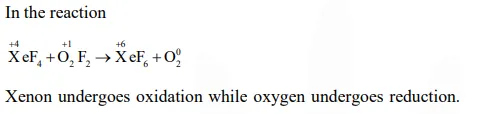Question: Which of the following reactions is an example of a redox reaction?
$\mathrm{XeF}_{4}+\mathrm{O}_{2} \mathrm{~F}_{2} \rightarrow \mathrm{XeF}_{6}+\mathrm{O}_{2}$
$\mathrm{XeF}_{2}+\mathrm{PF}_{5} \rightarrow\left[\mathrm{XeF}^{+} \mathrm{PF}_{6}^{-}\right.$
$\mathrm{XeF}_{6}+\mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{XeOF}_{4}+2 \mathrm{HF}$
$\mathrm{XeF}_{6}+2 \mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{XeO}_{2} \mathrm{~F}_{2}+4 \mathrm{HF}$
Correct Option: 1
Solution: