Question: Which one of the following reactions will not yield propionic acid?
$\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{COCH}_{3}+\mathrm{OI}^{-} / \mathrm{H}_{3} \mathrm{O}^{+}$
$\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{3}+\mathrm{KMnO}_{4}$ (Heat), $\mathrm{OH}^{-} / \mathrm{H}_{3} \mathrm{O}^{+}$
$\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CCl}_{3}+\mathrm{OH}^{-} / \mathrm{H}_{3} \mathrm{O}^{+}$
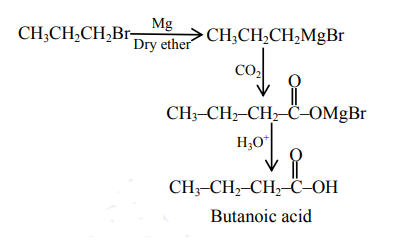
$\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{Br}+\mathrm{Mg}, \mathrm{CO}_{2}$ dry ether $/ \mathrm{H}_{3} \mathrm{O}^{+}$
Correct Option: , 4
Solution:
All gives propanoic acid as product but option 4 gives butanoic as product