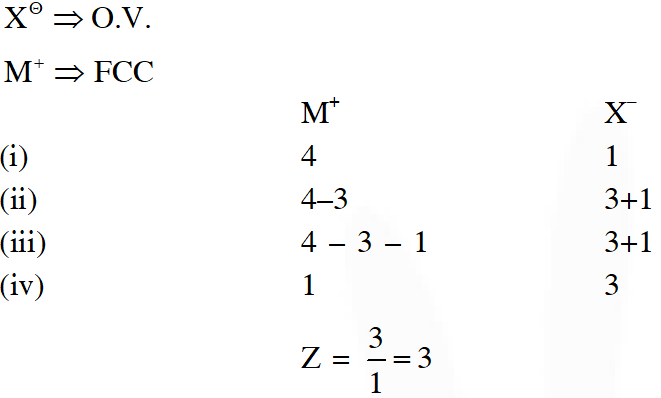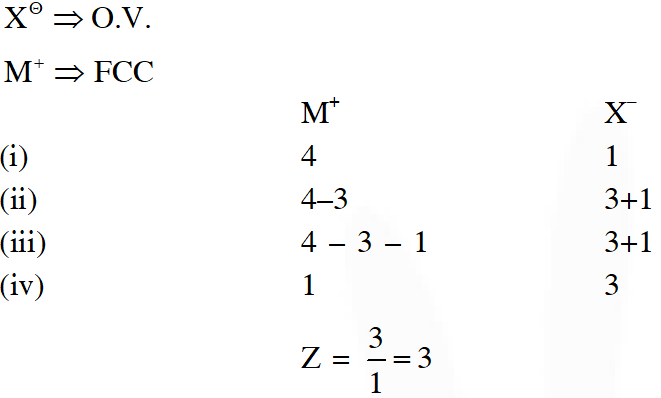JEE Advanced Previous Year Questions of Chemistry with Solutions are available at eSaral. Practicing JEE Advanced Previous Year Papers Questions of Chemistry will help the JEE aspirants in realizing the question pattern as well as help in analyzing weak & strong areas.
Simulator
Previous Years JEE Advance Questions
Q. The correct statement(s) regarding defects in solid is (are)
(A) Frenkel defect is usually favoured by a very small difference in the sizes of cation and
anion.
(B) Frenkel defect is a dislocation defect
(C) Trapping of an electron in the lattice leads to the formation of F‑center.
(D) Schottky defects have no effect on the physical properties of solids.
[JEE 2009]
Ans. (B,C)
Q. The packing effeciency of the two-dimensional square unit cell shown below is
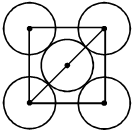 (A) 39.27% (B) 68.02% (C) 74.05% (D) 78.54%
[JEE-2010]
(A) 39.27% (B) 68.02% (C) 74.05% (D) 78.54%
[JEE-2010]
 (A) 39.27% (B) 68.02% (C) 74.05% (D) 78.54%
[JEE-2010]
(A) 39.27% (B) 68.02% (C) 74.05% (D) 78.54%
[JEE-2010]
Ans. (D)
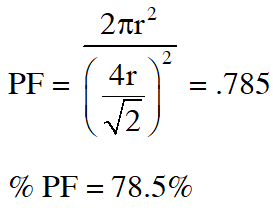
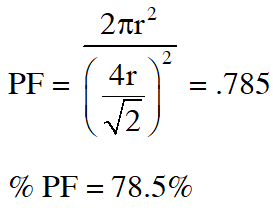
Q. The number of hexagonal faces that present in a truncated octahedron is.
[JEE-2011]
Ans. 8
Q. A compound $\mathrm{M}_{\mathrm{p}} \mathrm{X}_{\mathrm{q}}$ has cubic close packing (ccp) arrangement of X. Its unit cell structure is shown below. The empirical formula of the compound is :
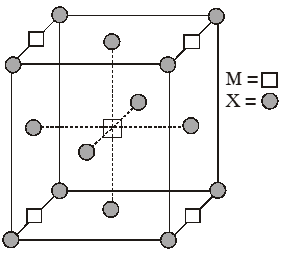 (A) MX
(B) $\mathrm{MX}_{2}$
(C) $\mathrm{M}_{2} \mathrm{X}$
(D) $\mathrm{M}_{5} \mathrm{X}_{14}$
[JEE-2012]
(A) MX
(B) $\mathrm{MX}_{2}$
(C) $\mathrm{M}_{2} \mathrm{X}$
(D) $\mathrm{M}_{5} \mathrm{X}_{14}$
[JEE-2012]
 (A) MX
(B) $\mathrm{MX}_{2}$
(C) $\mathrm{M}_{2} \mathrm{X}$
(D) $\mathrm{M}_{5} \mathrm{X}_{14}$
[JEE-2012]
(A) MX
(B) $\mathrm{MX}_{2}$
(C) $\mathrm{M}_{2} \mathrm{X}$
(D) $\mathrm{M}_{5} \mathrm{X}_{14}$
[JEE-2012]
Ans. (B)
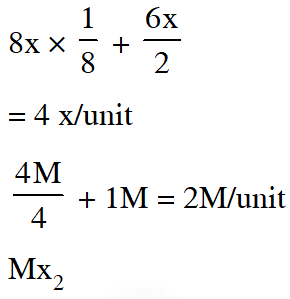
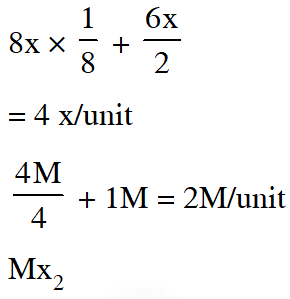
Q. The arrangement of $\mathrm{X}^{-}$ ions around $\mathrm{A}^{+}$ ion in solid AX is given in the figure (not drawn to scale). If the radius of $\mathrm{X}^{-}$ is 250 pm, the radius of $\mathrm{A}^{+}$ is -
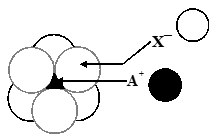 (A) 104 pm (B) 125 pm (C) 183 pm (D) 57 pm
[JEE-2013]
(A) 104 pm (B) 125 pm (C) 183 pm (D) 57 pm
[JEE-2013]
 (A) 104 pm (B) 125 pm (C) 183 pm (D) 57 pm
[JEE-2013]
(A) 104 pm (B) 125 pm (C) 183 pm (D) 57 pm
[JEE-2013]
Ans. (A)
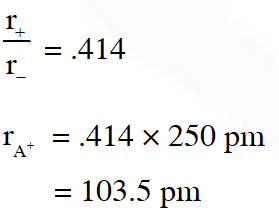
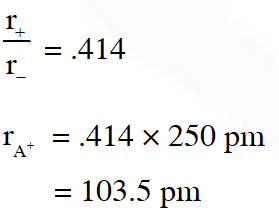
Q. The correct statement(s) for cubic close packed (ccp) three dimensional
structure is (are)
(A) The number of the nearest neighbours of an atom present in the topmost layer is 12
(B) The efficiency of atom packing is 74%
(C) The number of octahedral and tetrahedral voids per atom are 1 and 2, respectively
(D) The unit cell edge length is $2 \sqrt{2}$ times the radius of the atom
[JEE - Adv. 2016]
Ans. (B,C,D)
CCP is ABC ABC ..... type packing
(A) In topmost layer, each atom is in contact with 6 atoms in same layer and 3 atoms below
this layer.
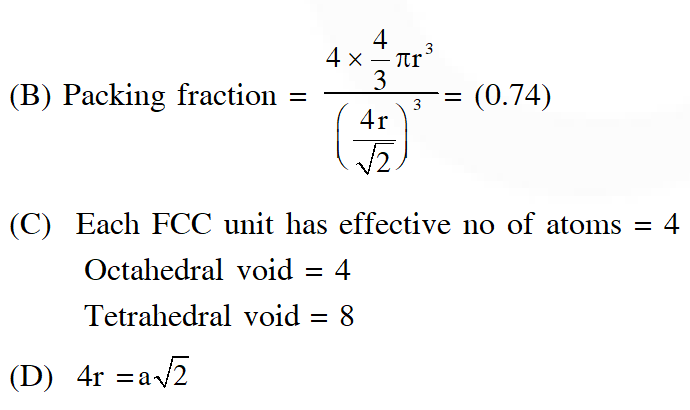
Q. A crystalline solid of a pure substance has a face-centred cubic structure with a cell edge of
400 pm. If the density of the substance in the crystal is 8g $\mathrm{cm}^{-3}$, then the number of atoms present in 256g of the crystal is $\mathrm{N} \times 10^{24}$. The value of N is
[JEE - Adv. 2017]
Ans. 2
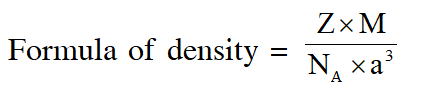
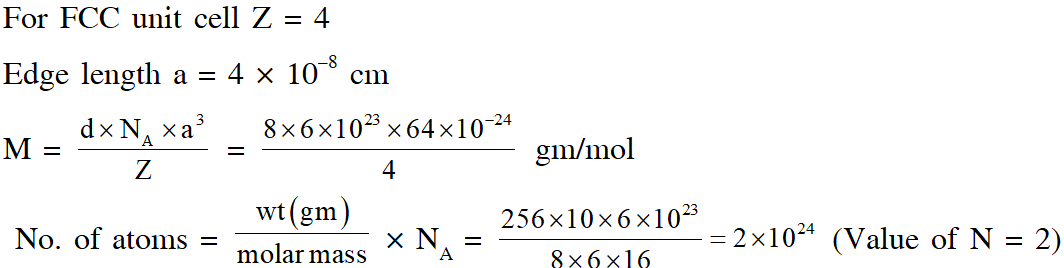
Q. Consider an ionic solid MX with NaCl structure. Construct a new structure (Z) whose unit cell is constructed from the unit cell of MX following the sequential instructions given below. Neglect the charge balance.
(i) Remove all the anions (X) except the central one
(ii) Replace all the face centered cations (M) by anions (X)
(iii) Remove all the corner cations (M)
(iv) Replace the central anion (X) with cation (M)
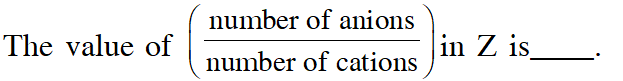 [JEE - Adv. 2017]
[JEE - Adv. 2017]
Ans. 3