JEE Advanced Previous Year Questions of Chemistry with Solutions are available at eSaral. Practicing JEE Advanced Previous Year Papers Questions of Chemistry will help the JEE aspirants in realizing the question pattern as well as help in analyzing weak & strong areas.
Simulator
Previous Years JEE Advance Questions
Q. Among the electrolytes $\mathrm{Na}_{2} \mathrm{SO}_{4}, \mathrm{CaCl}_{2}, \mathrm{Al}_{2}\left(\mathrm{SO}_{4}\right)_{3}$ and $\mathrm{NH}_{4} \mathrm{Cl}$, the most effective coagulation agent for $\mathrm{Sb}_{2} \mathrm{S}_{3}$ sol is
(A) $\mathrm{Na}_{2} \mathrm{SO}_{4}$
(B) $\mathrm{CaCl}_{2}$
(C) $\mathrm{Al}_{2}\left(\mathrm{SO}_{4}\right)_{3}$
(D) $\mathrm{NH}_{4} \mathrm{Cl}$
JEE 2009
Ans. (C)
$\mathrm{Sb}_{2} \mathrm{S}_{3}$ forms negatively charged sol. Hence cation with
greatest charge density is most effective in bringing about coagulation C.F. Hardy sulz rule.
Q. The correct statement(s) pertaining to the adsorption of a gas on a solid surface is
(are) -
(A) Adsorption is always exothermic
(B) Physisorption may transform into chemisorption at high temperature
(C) Physisorption increases with increasing temperature but chemisorption decreases with
increasing temperature
(D) Chemisorption is more exothermic than physisorption, however it is very slow due to higher energy of activation
JEE 2011
Ans. (A,B,D)
general properties.
Q. Choose the correct reason(s) for the stability of the lyophobic colloidal particle.
(A) Preferential adsorption of ions on their surface from the solution
(B) Preferential adsorption of solvent on their surface from the solution
(C) Attraction between different particles having opposite charges on their surface
(D) Potential difference between the fixed layer and the diffused layer of opposite charges around the colloidal particles
JEE 2012
Ans. (A,D)
Theory Based
Q. The given graphs / data I, II, III and IV represent general trends observed for different physisorption and chemisorption processes under mild conditions of temperature and pressure. Which of the following choice(s) about I, II, III and IV is (are) correct ?
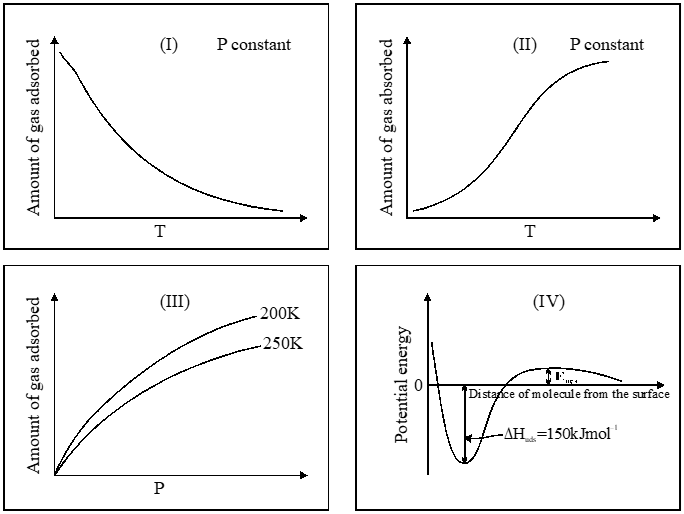 (A) I is physisorption and II is chemisorption
(B) I is physisorption and III is chemisorption
(C) IV is chemisorption and II is chemisorption
(D) IV is chemisorption and III is chemisorption
JEE 2012
(A) I is physisorption and II is chemisorption
(B) I is physisorption and III is chemisorption
(C) IV is chemisorption and II is chemisorption
(D) IV is chemisorption and III is chemisorption
JEE 2012
 (A) I is physisorption and II is chemisorption
(B) I is physisorption and III is chemisorption
(C) IV is chemisorption and II is chemisorption
(D) IV is chemisorption and III is chemisorption
JEE 2012
(A) I is physisorption and II is chemisorption
(B) I is physisorption and III is chemisorption
(C) IV is chemisorption and II is chemisorption
(D) IV is chemisorption and III is chemisorption
JEE 2012
Ans. (A,C)
For physical adsorption $\rightarrow$ favourable conditions is decrease in temp.
for chemical adsorption $\rightarrow$ chemical bond
Occurs $\rightarrow$ PE $\downarrow$ with bonding and increase in temp..
Q. Methylene blue, from its aqueous solution, is adsorbed on activated charcoal at $25^{\circ} \mathrm{C}$. For this process, the correct statement is
(A) The adsorption requires activation at $25^{\circ} \mathrm{C}$
(B) The adsorption is accompanied by a decrease in enthalpy
(C) The adsorption increases with increase of temperature
(D) The adsorption is irreversible
JEE Adv. 2013
Ans. (B)
Explanation for physical adsorption
• Activation energy is very low
• Physical adsorption is an exothermic process
• Physical adsorption decreases with increase in temperature
• Physical adsorption is reversible
Q. The qualitative sketches I , II and III given below show the variation of surface tension with molar concentration of three different aqueous solutions of KCl, $\mathrm{CH}_{3} \mathrm{OH}$ and $\mathrm{CH}_{3}\left(\mathrm{CH}_{2}\right)_{11} \mathrm{OSO}_{3}^{-} \mathrm{Na}^{+}$ at room temperature. The correct assignment of the sketches is –
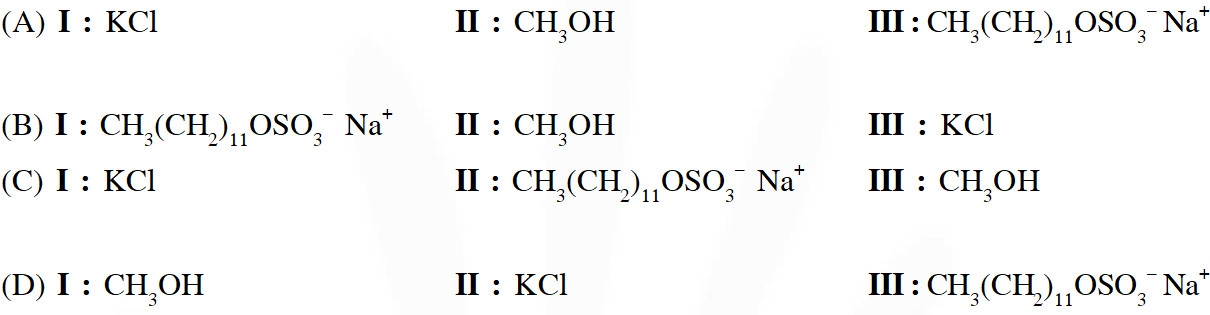 JEE Adv. 2016
JEE Adv. 2016
 JEE Adv. 2016
JEE Adv. 2016
Ans. (D)
Water has large surface tension due to very strong interaction. Generally adding organic
derivatives to water decreases its surface tension due to hydrophobic interaction.
In case III, hydrophobic interaction is stronger than case I causing surface tension to decrease more rapidly.
Q. The correct statement(s) about surface properties is (are)
(A) Cloud is an emulsion type of colloid in which liquid is dispersed phase and gas is dispersion
medium
(B) Adsorption is accompanied by decrease in enthalpy and decrease in entropy of the system.
(C) Brownian motion of colloidal particles does not depend on the size of the particles but
depends on viscosity of the solution.
(D) The critical temperatures of ethane and nitrogen and 563 K and 126 K, respectively. The adsorption of ethane will be more than that of nitrogen on same amount of activated charcoal at a given temperature.
JEE Adv. 2017
Ans. (B,D)
(A) Emulsion is liquid in liquid type colloid.
(B) For adsorption, $\Delta \mathrm{H}<0 \& \Delta \mathrm{S}<0$
(C) Smaller the size and less viscous the dispersion medium, more will be the brownian motion.
(D) Higher the $\mathrm{T}_{\mathrm{C}}$ , greater will be the extent of adsorption.
Comments
shrutiiiii
Sept. 25, 2020, 11:16 p.m.
good work but need some more updates..........btw it helped me...
