JEE Main Previous Year Papers Questions of Chemistry with Solutions are available at eSaral. Practicing JEE Main chapter wise questions of Chemistry will help the JEE aspirants in realizing the question pattern as well as help in analyzing weak & strong areas.
Simulator
Previous Years AIEEE/JEE Main Questions
Q. Which of the following statements is incorrect regarding physisorptions ?
(1) Under high pressure it results into multi molecular layer on adsorbent surface
(2) Enthalpy of adsorption $\left(\Delta \mathrm{H}_{\text {adsorption }}\right)$ is low and
positive
(3) It occurs because of Van der Waal's forces
(4) More easily liquefiable gases are adsorbed readi
AIEEE-2009
Ans. (2)
$\Delta \mathrm{H}$ is negative
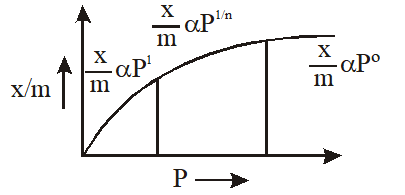
Q. According to Freundlich adsorption isotherm, which of the following is correct ?
(1) $\frac{\mathrm{x}}{\mathrm{m}} \propto \mathrm{p}^{0}$
(2) $\frac{\mathrm{x}}{\mathrm{m}} \propto \mathrm{p}^{1}$
(3) $\frac{\mathrm{x}}{\mathrm{m}} \propto \mathrm{p}^{1 / \mathrm{n}}$
(4) All the above are correct for different ranges of pressure
AIEEE-2012
Ans. (4)

Q. The coagulating power of electrolytes having ions $\mathrm{Na}^{+}, \mathrm{Al}^{3+}$ and $\mathrm{Ba}^{2+}$ for aresenic sulphide sol increases in the order :-
(1) $\mathrm{Al}^{3+}<\mathrm{Ba}^{2+}<\mathrm{Na}^{+}$
(2) $\mathrm{Na}^{+}<\mathrm{Ba}^{2+}<\mathrm{Al}^{3+}$.
(3) $\mathrm{Ba}^{2+}<\mathrm{Na}^{+}<\mathrm{Al}^{3+}$
(4) $\mathrm{Al}^{3+}<\mathrm{Na}^{+}<\mathrm{Ba}^{2+}$
JEE-Main 2013
Ans. (2)
According to hardley schuzle rule
Q. For a linear plot of log(x/m) versus log p in a Freundlich adsorption isotherm, which of the following statements is correct ? (k and n are constants)
(1) log (1/n) appears as the intercept
(2) Both k and 1/n appear in the slope term
(3) 1/n appears as the intercept
(4) Only 1/n appears as the slope
JEE-Main 2016
Ans. (4)
According to Freundlich isotherm
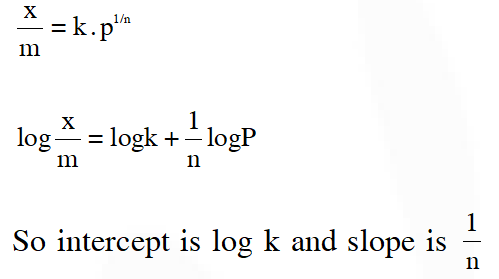
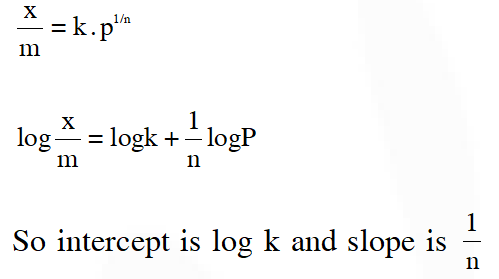
Q. The Tyndall effect is observed only when following conditions are satisfied :-
(a) The diameter of the dispersed particles is much smaller than the wavelength of the ligh
used.
(b) The diameter of the dispersed particle is not much smaller than the wavelength of the light
used.
(c) The refractive indices of the dispersed phase and dispersion medium are almost similar in
magnitude.
(d) The refractive indices of the dispersed phase and dispersion medium differ greatly in
magnitude.
(1) (a) and (d)
(2) (b) and (d)
(3) (a) and (c)
(4) (b) and (c)
JEE - Main - 2017
Ans. (2)
As per NCERT book (fact)
