Get Surface Chemistry important questions for Boards exams. Download or View the Important Question bank for Class 12 Chemistry. These important questions will play significant role in clearing concepts of Chemistry. This question bank is designed by NCERT keeping in mind and the questions are updated with respect to upcoming Board exams. You will get here all the important questions for class 12 chemistry chapter wise CBSE.
Click Here for Detailed Chapter-wise Notes of Chemistry for Class 12th, JEE & NEET.
You can access free study material for all three subject’s Physics, Chemistry and Mathematics.
Click Here for Detailed Notes of any chapter.
eSaral provides you complete edge to prepare for Board and Competitive Exams like JEE, NEET, BITSAT, etc.
We have transformed classroom in such a way that a student can study anytime anywhere. With the help of AI we have made the learning Personalized, adaptive and accessible for each and every one.
Visit eSaral Website to download or view free study material for JEE & NEET.
Also get to know about the strategies to Crack Exam in limited time period.
Q.
Why are substances like platinum and palladium often used for carrying out electrolysis of aqueous solutions?
Ans. Platinum and palladium form inert electrodes, i.e., they are not attacked by the ions of the electrolyte or the products of electrolysis. Hence, they are used as electrodes for carrying out the electrolysis.
Q. Why are powdered substances more effective adsorbent than their crystalline forms ?
Ans. Powdered substances have greater surface area as compared to their crystalline forms. Greater the surface area, greater is the adsorption.
Q. Why is it necessary to remove CO when ammonia is obtained by Haber’s process ?
Ans. CO acts as a poison for the catalyst used in the manufacture of ammonia by Haber’s process. Hence, it is necessary to remove it.
Q. Why is the ester hydrolysis slow in the beginning and becomes faster after some time ?
Ans. The ester hydrolysis takes place as follows :
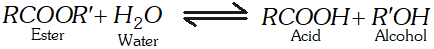 The acid produced in the reaction acts as catalyst (autocatalyst) for the reaction. Hence, the reaction becomes faster after some time.
The acid produced in the reaction acts as catalyst (autocatalyst) for the reaction. Hence, the reaction becomes faster after some time.
Q. What is the role of desorption in the process of catalysis?
Ans. Desorption makes the surface of the solid catalyst free for fresh adsorption of the reactants on the surface.
Q. Give reason why a finely divided substance is more effective as an adsorbent ?
Ans. Adsorption is a surface phenomenon. Since finely divided substance has large surface area, hence, adsorption occurs to a greater extent.
Q. What is demulsification? Name two demulsifiers.
Ans. The decomposition of an emulsion into constituent liquids is called demulsification.
Demulsification can be done by boiling or freezing.
Q. What do you understand by activity and selectivity of catalysts.
Ans. Activity of a catalyst refers to the ability of catalyst to increase the rate of chemical reaction. Selectivity of a catalyst refers to its ability to direct the reaction to give a specific product.
Q. Why does physisorption decrease with increase of temperature ?
Ans. Physisorption is an exothermic process :
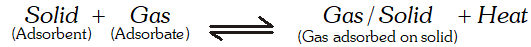 According to Le Chatelier's principle, if we increase the temperature, equilibrium will shift in the backward direction, i.e., gas is released from the adsorbed surface.
According to Le Chatelier's principle, if we increase the temperature, equilibrium will shift in the backward direction, i.e., gas is released from the adsorbed surface.
Q. What modification can you suggest in the Hardy Schulze law ?
Ans. According to Hardy Schulze law, the coagulating ion has charge opposite to that on the colloidal particles. Hence, the charge on colloidal particles is neutralized and coagulation occurs. The law can be modified to include the following :
When oppositely charged sols are mixed in proper proportions to neutralize the charges of each other, coagulation of both the sols occurs.
Q. Why is it essential to wash the precipitate with water before estimating it quantitatively ?
Ans. Some amount of the electrolytes mixed to form the precipitate remains adsorbed on the surface of the particles of the precipitate. Hence, it is essential to wash the precipitate with water to remove the sticking electrolytes (or any other impurities) before estimating it quantitatively.
Q. Distinguish between the meaning of the terms adsorption and absorption. Give one example of each.
Ans. In adsorption, the substance is concentrated only at surface and does not penetrate through the surface to the bulk of adsorbent while in absorption, the substance is uniformly distributed through out the bulk of the solid.
A distinction can be made between absorption and adsorption by taking an example of water vapour. Water vapours are absorbed by anhydrous calcium chloride but adsorbed by silica gel.
Q. What role does adsorption play in heterogeneous catalysis?
Ans. In heterogeneous catalysis, the reactants are generally gases whereas catalyst is a solid. The reactant molecules are adsorbed on the surface of the solid catalyst by physical adsorption or chemisorption. As a result, the concentration of the reactant molecules on the surface increases and hence the rate of reaction increase. Alternatively, one of the reactant molecules undergo fragmentation on the surface of the solid catalyst producing active species which react faster. The product molecules in either case have no affinity for the solid catalyst and are desorbed making the surface free for fresh adsorption. This theory is called adsorption theory.
Q. Give two chemical methods for the preparation of colloids.
Ans. Colloidal solutions can be prepared by chemical reactions involving, double decomposition, oxidation, reduction and hydrolysis.
(i) Double decomposition : A colloidal sol of arsenious sulphide is obtained by passing hydorgen sulphide into a solution of arsenious oxide in distilled water.
$A s_{2} O_{3}+3 H_{2} S \rightarrow A s_{2} S_{3}+3 H_{2} O$
(ii) Oxidation : A colloidal solution of sulphur can be obtained by passing hydrogen sulphide into a solution of sulphur dioxide in water through a solution of an oxidizing agent (bromide water, nitric acid etc.)
$\mathrm{SO}_{2}+2 \mathrm{H}_{2} \mathrm{S} \rightarrow 3 \mathrm{S}+2 \mathrm{H}_{2} \mathrm{O}$
$H_{2} S+[O] \rightarrow H_{2} O+S$
Q. How are the colloidal solutions classified, on the basis of physical states of the dispersed phase and dispersion medium ?
Ans.
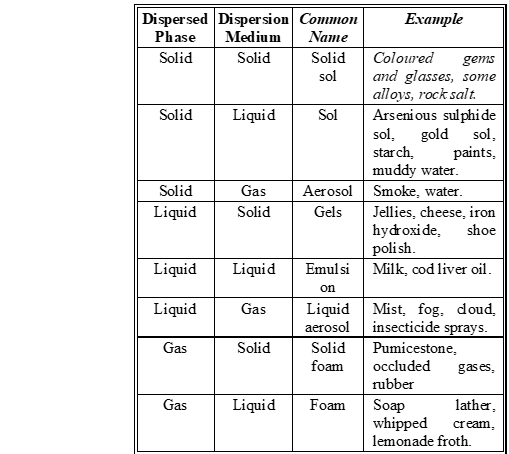

Q. Describe a chemical method each for the preparation of sol of sulphur and platinum in water.
Ans. Preparation of sol of sulphur : A colloidal solution o sulphur can be obtained by passing hydrogen sulphide into a solution of sulphur dioxide in water or through solution of an oxidizing agent (bromine water, nitric acid etc.)
$\mathrm{SO}_{2}+2 \mathrm{H}_{2} \mathrm{S} \longrightarrow 3 \mathrm{S}+\mathrm{H}_{2} \mathrm{O}$
$H_{2} S+[O] \longrightarrow H_{2} O+[S]$
Preparation of platinum sol : It is prepared by electrical disintegration or Bredig’s arc method. In this method, electric arc is struck between electrodes of the metal immersed in the dispersion medium. The intense heat produced vaporizes some of the metal, which then condenses to form particles of calloidal size.
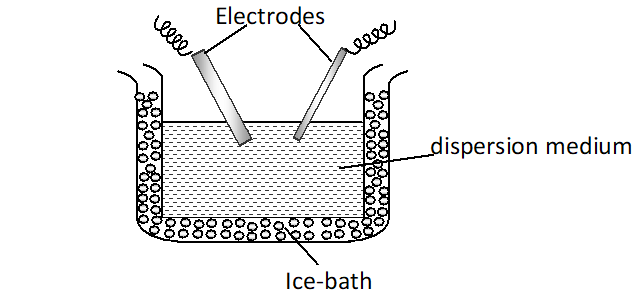

Q. Give four examples of heterogeneous catalysis.
Ans. When the catalyst exists in a different phase than that of reactants. It is said to be heterogeneous catalyst and the catalysis is called heterogeneous catalysis.
(i) Manufacture of $N H_{3}$ from $N_{2}$ and $H_{2}$ by Haber's process using iron as catalyst.
$N_{2}+3 H_{2} \stackrel{F e}{\longrightarrow} 2 N H_{3}$
(ii) Manufacture of $\mathrm{CH}_{3} \mathrm{OH}$ from $\mathrm{CO}$ and using $(\mathrm{a}$ mixture of $C u, Z n O$ and $C r_{2} O_{3}$
$\mathrm{CO}+2 \mathrm{H}_{2} \frac{\mathrm{Cu}+\mathrm{ZnO}+\mathrm{Cr}_{2} \mathrm{O}_{3}}{\mathrm{Heat}} \rightarrow \mathrm{CH}_{3} \mathrm{OH}$
(iii) Oxidation of with using as catalyst in ostwald process.
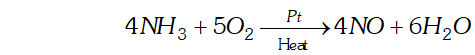 (iv) Hydrogenation of oils to form vegetable ghee using finely divided nickel.
$\mathrm{Oil}+\mathrm{H}_{2} \stackrel{\mathrm{Ni}}{\longrightarrow}$ Vegetable ghee.
(iv) Hydrogenation of oils to form vegetable ghee using finely divided nickel.
$\mathrm{Oil}+\mathrm{H}_{2} \stackrel{\mathrm{Ni}}{\longrightarrow}$ Vegetable ghee.
Q. Describe some features of catalysis by zeolites
Ans. Features of catalysis by zeolites. (i) Zeolites are hydrated alumino-silicates which have a three-dimensional network structure containing water molecules in their pores. (ii) To use them as catalyst, they are heated so that water of hydration present in the pores is lost and the pores become vacant. (iii) The size of the pores varies from 260 to $740 \mathrm{pm} .$ Thus, only those molecules adsorbed in these pores and catalysed whose size is small enough to enter these pores hence, they act as molecular sieves or shape selective catalyst. An important catalyst used in petroleum industry is ZSM- 5 (Zeolite sieve of molecular porosity 5 ). It converts alcohol into petrol by first dehydrating them to form a mixture of hydrocarbons.
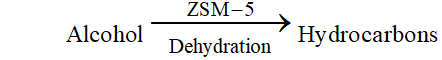
Q. What is shape selective catalysis.
Ans. The catalytic reaction that depends upon the pore structure of the catalyst and the size of the reactant and product molecules is called shape-selective catalysis. Zeolites are good shape-selective catalysts because of their honeycomb-like structures. They are microporous aluminosilicates with three dimensional network of silicates in which some silicon atoms are replaced by aluminium atoms giving $A l-O-S i$ framework. The reactions taking place in zeolites depend upon the size and shape of reactant and product molecules as well as upon the pores and cavities of the zeolites. They are found in nature as well as synthesised for catalytic selectivity.
Zeolites are being very widely used as catalysts in petrochemical industries for cracking of hydrocarbons and isomerisation. An important zeolite catalyst used in the petroleum industry is ZSM- - . It converts alcohols directly into gasoline (petrol) by dehydrating them to give a mixture of hydrocarbons.
Q. Give four uses of emulsions.
Ans. Uses of emulsions are :
(1) In medicines : A wide variety of pharmaceutical preparations are emulsions. For example, emulsions of cod liver oil. These emulsified oils are easily acted upon by digestive juices in the stomach and, hence are readily digested.
(2) Digestion of fats : Digestion of fats in the intestines is aided by emulsification.
(3) In disinfectants : The disinfectants such as dettol give emulsions of the oil in water type when mixed with water.
(4) In building roads : An emulsion of asphalt and water is used for building roads. In this way there is no necessity of melting the asphalt.
Q. What are micelles ? Give an example of a micellers system.
Ans. There are some substances which at low concentrations behave as normal strong electrolytes, but at higher concentration exhibit colloidal behaviour due to the formation of aggregates. The aggregated Particles thus formed are called micelles. The formation of micelles takes place only above a particular temperature called kraft temperature $\left(\mathrm{T}_{\mathrm{k}}\right)$ and above a particular concentr-ation called critical micelle concentration (CMC). Surface active agents such as soaps and synthetic detergents belong to this class. For soaps, the CMC is $10^{-4}$ to $10^{-3} \mathrm{moll}^{-1}$. micelles may contain as many as 100 molecules or more.
Q. Comment on the statement that “Colloid is not a substance but state of a substance”.
Ans. The given statement is true. This is because the same substance may exist as a colloid under certain conditions and as a crystalloid under certain other conditions. For example, $N a C l$ in water behaves as a crystalloid while in benzene, it behaves as a colloid. Similarly, dilute soap solution behaves like a crystalloid while concentrated solution behaves as a colloid (called associated colloid). It is the size of the particles which matters, i.e., the state in which the substance exists. If the size of the particles lies in the range 1 nm to 1000 nm to it is in the colloidal state.
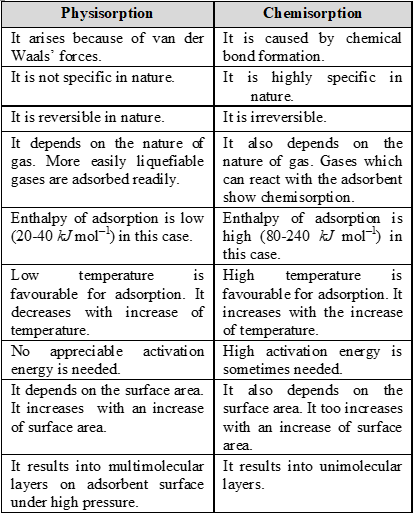
Q. What is the difference between physical adsorption and chemisorption?
Ans.

Q. What are the factors which influence the adsorption of gas on a solid?
Ans. Factors influencing the adsorption of gas on solid:
(i) Nature of gas: Under given conditions of temperature and pressure, the easily liquifiable gases like $N H_{3}, H C l, C O_{2},$ etc. are adsorbed to a greater extent than
the permanent gases such as $H_{2}, O_{2}, N_{2}$ etc. It is because the vander waal's forces or molecular forces are more predominant in the former than in later category.
Effect of Nature of the Adsorbent: Activated charcoal (ii) is the most common adsorbent for the gases which can be easily liquefied. The gases $H_{2}, O_{2}$ and $N_{2}$ are adsorbed on metals like $N i, P d$ etc.
(iii) Specific area of the solid : Specific area of an adsorbing solid is the surface available for adsorption per gram of the adsorbent. Greater the specific area of the solid, greater would be its adsorbent power.
(vi) Effect of pressure : It is expected that extent of adsorption increases with increase of pressure.
(v) Effect of temperature : In physisorption
$x / m$ decreases with rise in temperature. While in chemisorptionslightly increases in the beginning and then decreases as the temperature rises. This initial increase is due to the fact that, like chemical reactions, chemisorption also requires activation energy.
Q. What is an adsorption isotherm? Distinguish between Freundlich adsorption isothermal and Langmuir adsorption isotherm.
Ans. Adsorption isotherm. It is a graph indicating the variation of the mass or the gas adsorbed per gram $(x / m)$ of the adsorbent with pressure $(p)$ at constant temperature. Distinction between Freundlich and Langmuir adsorption isotherms
(i) The mathematical expressions representing adsorption are:
Freundlich adsorption isotherm: $\frac{x}{m}=k p^{1 / n}$
Langmuir adsorption isotherm: $\frac{x}{m}=\frac{a p}{1+b p}$
(ii) Langmuir adsorption isotherm is more general and is applicable at all pressure while Freundlich adsorption isotherm fails at high pressure. The latter can be deduced from the former.
(iii) Langmuir adsorption postulates that adsorption is monomolecular whereas Freundlich adsorption suggests that it is multimolecular.
Q. What do you understand by activation of adsorbent? How is it achieved?
Ans. Activation of adsorbent implies increases in the adsorption power of the adsorbent. It involves increase in the surface area of the adsorbent and is achieved by following methods.
$\bullet$ By finely dividing the adsorbent.
$\bullet$ By removing the gases already adsorbed
$\bullet$ By making the surface of adsorbent rough by chemical or mechanical methods.
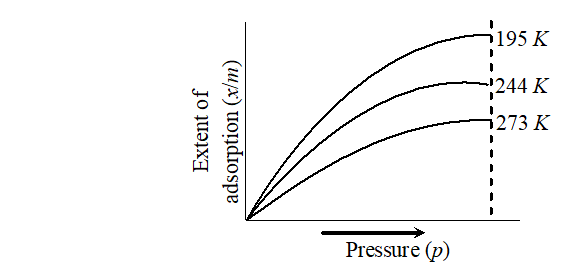
Q. Discuss the effect of pressure and temperature on the adsorption of gases by solids.
Ans. Effect of pressure : It is expected that extent of adsorption increases with increase in pressure. The extent of the adsorption is generally expressed as $x / m$ where $m$ is the mass of the adsorbent and $x$ is that of the adsorbate when equilibrium has been attained. A graph drawn between
extent of adsorption $\left(\frac{x}{m}\right)$ and the pressure $p$ of the gas at constant temperature is called adsorption isotherm.
 . Effect of temperature : Magnitude of adsorption decrease with increase in the temperature.
A graph drawn between extent of adsorption $\left(\frac{x}{m}\right)$ and temperature ( $T$ ) at constant pressure is called adsorption isobar physical adsorption isobar shows a decrease in If with rise in temperature, the isobar of chemisorption $x / 1$ shows an increase in the beginning and then decreases as the temperature rises. This initial increase is due to the fact that like chemical reactions, chemisorption also requires activation energy.
. Effect of temperature : Magnitude of adsorption decrease with increase in the temperature.
A graph drawn between extent of adsorption $\left(\frac{x}{m}\right)$ and temperature ( $T$ ) at constant pressure is called adsorption isobar physical adsorption isobar shows a decrease in If with rise in temperature, the isobar of chemisorption $x / 1$ shows an increase in the beginning and then decreases as the temperature rises. This initial increase is due to the fact that like chemical reactions, chemisorption also requires activation energy.
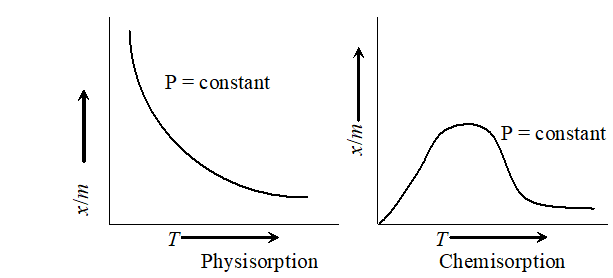
 . Effect of temperature : Magnitude of adsorption decrease with increase in the temperature.
A graph drawn between extent of adsorption $\left(\frac{x}{m}\right)$ and temperature ( $T$ ) at constant pressure is called adsorption isobar physical adsorption isobar shows a decrease in If with rise in temperature, the isobar of chemisorption $x / 1$ shows an increase in the beginning and then decreases as the temperature rises. This initial increase is due to the fact that like chemical reactions, chemisorption also requires activation energy.
. Effect of temperature : Magnitude of adsorption decrease with increase in the temperature.
A graph drawn between extent of adsorption $\left(\frac{x}{m}\right)$ and temperature ( $T$ ) at constant pressure is called adsorption isobar physical adsorption isobar shows a decrease in If with rise in temperature, the isobar of chemisorption $x / 1$ shows an increase in the beginning and then decreases as the temperature rises. This initial increase is due to the fact that like chemical reactions, chemisorption also requires activation energy.

Q. What are lyophilic and lyophobic sols ? give one example of each type.
Ans. (i) Lyophilic colloids : The word ‘lyophilic’ means liquid-loving. Colloidal sols directly formed by mixing substances like gum, gelatine, starch, rubber, etc., with a suitable liquid (the dispersion medium) are called lyophilic sols. An important characteristic of these sols is that if the dispersion medium is separated from the dispersed phase (say by evaporation), the sol can be reconstituted by simply remixing with the dispersion medium. That is why these sols are also called reversible sols. Furthermore, these sols are quite stable and cannot be easily coagulated.
(ii) Lyophobic colloids : The word ‘lyophobic’ means liquid-hating. Substances like metals, their sulphides, etc., when simply mixed with the dispersion medium do not form the colloids. Their colloidal sols can be prepared only by special methods. Such sols are called lyophobic sols. These sols are readily precipitated (or coagulated) on the addition of small amounts of electrolytes, by heating or by shaking and hence, are not stable. Further, once precipitated, they do not give back the colloidal sol by simple addition of the dispersion medium. Hence, these sols are also called irreversible sols. Lyophobic sols need stabilising agents for their preservation.
Q. What is the difference between multimolecular and macromolecular colloids ? Give one example of each. How are associated colloids different from these two types of colloids ?
Ans. On dissolution, a large number of atoms or smaller mole-cules of a substance aggregate together to form species having size in the colloidal range (diameter < 1 nm). The species thus formed are multimolecular colloids while macromolecules in suitable solvents form solutions in which the size of macromolecules may be in colloidal range.
Difference between associated colloids multimolecular and macromolecules colloids. Multimolecular colloids are formed by the aggregation of a large number of small atoms molecules such as S8. Macromolecular colloids contain molecules of large size like starch the size of molecules in this case have the dimensions of colloids. Their size lies in the colloidal range. The associated colloids are formed by electrolytes which dissociate into ions and these ions associate together to form ionic micelles whose size lies in the colloidal range, soap is common example of this type . The micelle formation occurs above a particular concentration (called critical micellisation concentration) and above a particular temperature, called Kraft temperature.
Q. Explain what is observed when
(i) An electrolyte is added to ferric hydroxide sol
(ii) An emulsion is subjected to centrifugation
(iii) Direct current is passed through a colloidal sol
(iv) A beam of light is passed through a colloidal solution.
Ans. (i) The positively charged colloidal particles of $F e(O H)_{3}$ sol get coagulated by the oppositely charged ion provided by electrolyte.
(ii) The constituent liquids of the emulsion separate out. In other words, demulsification occurs.
(iii) On passing direct current, colloidal particles move towards the oppositely charged electrode where they lose their charge and get coagulated.
(iv) Scattering of light by the colloidal particles takes place and the path of light becomes illuminated. This is called Tyndall effect.
Q. Explain the following terms :
(1) Peptization, (2) Electrophoresis, (3) Coagulation,
Ans. (1) Peptization : Peptization may be defined as the process of converting a precipitate into colloidal sol by shaking it with dispersion medium in presence of a small amount of electrolyte. The electrolyte used for this purpose is called peptizing agent.
(2) Electrophoresis : In this process colloidal particles move towards oppositely charged electrodes, get discharged and precipitated.
(3) Coagulation : The stability of the lyophobic sol is due to the presence of charge on colloidal particles. If, some how, the charge is removed, the particles will come nearer to each other to form aggregate (or coagulate) and settle down.
Q. Explain the following terms with suitable examples
(1) Gel (2) Aerosol and (3) Hydrosol
Ans. (1) Gel : It is a colloidal dispersion of a liquid in a solid, common example is butter.
(2) Aerosol : It is a colloidal dispersion of a liquid in a gas, common example is, fog.
(3) Hydrosol :It is a colloidal sol of a solid in water as the dispersion medium, common example is starch sol or gold sol.
Q. What are emulsions ? What are their different types ? Give one example of each type.
Ans. These are liquid-liquid colloidal systems, i.e., the dispersion of finely divided droplets in another liquid. If a mixture of two immiscible or partially miscible liquids is shaken, a coarse dispersion of one liquid in the other is obtained which is called emulsion. Generally, one of the two liquids is water. There are two types of emulsions.
(i) Oil dispersed in water (O/W type) and
(ii) Water dispersed in oil (W/O type ).
In the first system, water acts as dispersion medium. Examples of this type of emulsion are milk and vanishing cream. In milk, liquid fat is dispersed in water. In the second system, oil acts as dispersion medium. Common examples of this type are butter and cream.
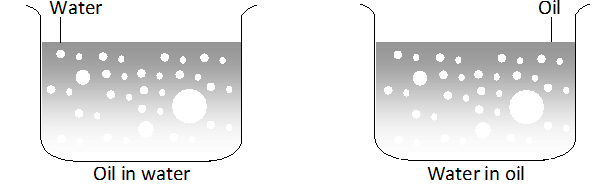 Emulsions of oil in water are unstable and sometimes they separate into two layers on standing. For stabilisation of an emulsion, a third component called emulsifying agent is usually added. The emulsifying agent forms an interfacial film between suspended particles and the medium. The principal emulsifying agents of O/W emulsions are proteins, gums, natural and synthetic soaps, etc., and for W/O, heavy metal, salts of fatty acids, long chain alcohols, etc.
Emulsions can be diluted with any amount of the dispersion medium. On the other hand, the dispersed liquid when mixed, forms a separate layer. The droplets in emulsions are often negatively charged and can be precipitated by electrolytes. They also show Brownian movement and Tyndall effect. Emulsions can be broken into constituent liquids by heating, freezing, etc.
Emulsions of oil in water are unstable and sometimes they separate into two layers on standing. For stabilisation of an emulsion, a third component called emulsifying agent is usually added. The emulsifying agent forms an interfacial film between suspended particles and the medium. The principal emulsifying agents of O/W emulsions are proteins, gums, natural and synthetic soaps, etc., and for W/O, heavy metal, salts of fatty acids, long chain alcohols, etc.
Emulsions can be diluted with any amount of the dispersion medium. On the other hand, the dispersed liquid when mixed, forms a separate layer. The droplets in emulsions are often negatively charged and can be precipitated by electrolytes. They also show Brownian movement and Tyndall effect. Emulsions can be broken into constituent liquids by heating, freezing, etc.
 Emulsions of oil in water are unstable and sometimes they separate into two layers on standing. For stabilisation of an emulsion, a third component called emulsifying agent is usually added. The emulsifying agent forms an interfacial film between suspended particles and the medium. The principal emulsifying agents of O/W emulsions are proteins, gums, natural and synthetic soaps, etc., and for W/O, heavy metal, salts of fatty acids, long chain alcohols, etc.
Emulsions can be diluted with any amount of the dispersion medium. On the other hand, the dispersed liquid when mixed, forms a separate layer. The droplets in emulsions are often negatively charged and can be precipitated by electrolytes. They also show Brownian movement and Tyndall effect. Emulsions can be broken into constituent liquids by heating, freezing, etc.
Emulsions of oil in water are unstable and sometimes they separate into two layers on standing. For stabilisation of an emulsion, a third component called emulsifying agent is usually added. The emulsifying agent forms an interfacial film between suspended particles and the medium. The principal emulsifying agents of O/W emulsions are proteins, gums, natural and synthetic soaps, etc., and for W/O, heavy metal, salts of fatty acids, long chain alcohols, etc.
Emulsions can be diluted with any amount of the dispersion medium. On the other hand, the dispersed liquid when mixed, forms a separate layer. The droplets in emulsions are often negatively charged and can be precipitated by electrolytes. They also show Brownian movement and Tyndall effect. Emulsions can be broken into constituent liquids by heating, freezing, etc.
Q. Action of soap is due to emulsification and micelle formation. Comment.
Ans. Cleansing action of soap: Washing action of soap is due to the emulsification of grease and taking it away in the water along with dirt or dust present on grease.
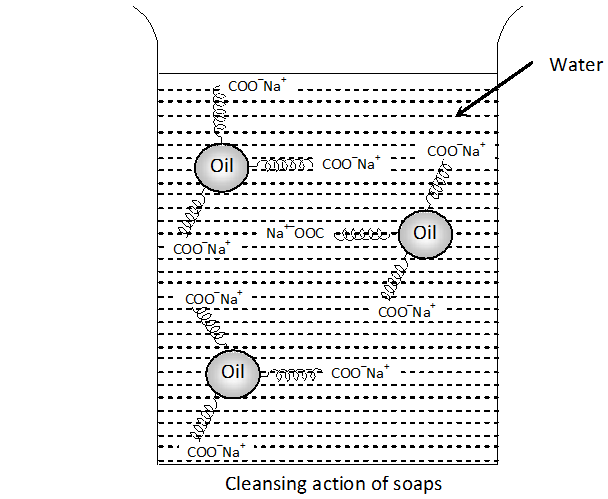 Explanation : The cleansing action of soap can be explained keeping in mind that a soap molecule contains a non-polar hydrophobic group and a polar hydrophilic group. The dirt is held on the surface of clothes by the oil or grease which is present there. since oil or grease are not soluble in water, therefore, the dirt particles cannot be removed by simply washing the cloth with water. When soap is applied, the non-polar alkyl group dissolves in oil droplets while the polar $-C O O^{-} N a^{+}$ groups remain dissolved in water (Figure). In this way, each oil droplet is surrounded by negative charge. These negatively charged oil droplets cannot coagulate and a stable emulsion is formed. These oil droplets (containing dirt particles) can be washed away with water along with dirt particles.
Explanation : The cleansing action of soap can be explained keeping in mind that a soap molecule contains a non-polar hydrophobic group and a polar hydrophilic group. The dirt is held on the surface of clothes by the oil or grease which is present there. since oil or grease are not soluble in water, therefore, the dirt particles cannot be removed by simply washing the cloth with water. When soap is applied, the non-polar alkyl group dissolves in oil droplets while the polar $-C O O^{-} N a^{+}$ groups remain dissolved in water (Figure). In this way, each oil droplet is surrounded by negative charge. These negatively charged oil droplets cannot coagulate and a stable emulsion is formed. These oil droplets (containing dirt particles) can be washed away with water along with dirt particles.
 Explanation : The cleansing action of soap can be explained keeping in mind that a soap molecule contains a non-polar hydrophobic group and a polar hydrophilic group. The dirt is held on the surface of clothes by the oil or grease which is present there. since oil or grease are not soluble in water, therefore, the dirt particles cannot be removed by simply washing the cloth with water. When soap is applied, the non-polar alkyl group dissolves in oil droplets while the polar $-C O O^{-} N a^{+}$ groups remain dissolved in water (Figure). In this way, each oil droplet is surrounded by negative charge. These negatively charged oil droplets cannot coagulate and a stable emulsion is formed. These oil droplets (containing dirt particles) can be washed away with water along with dirt particles.
Explanation : The cleansing action of soap can be explained keeping in mind that a soap molecule contains a non-polar hydrophobic group and a polar hydrophilic group. The dirt is held on the surface of clothes by the oil or grease which is present there. since oil or grease are not soluble in water, therefore, the dirt particles cannot be removed by simply washing the cloth with water. When soap is applied, the non-polar alkyl group dissolves in oil droplets while the polar $-C O O^{-} N a^{+}$ groups remain dissolved in water (Figure). In this way, each oil droplet is surrounded by negative charge. These negatively charged oil droplets cannot coagulate and a stable emulsion is formed. These oil droplets (containing dirt particles) can be washed away with water along with dirt particles.
