Hey, do you want to know what is Surface tension in physics? If so. Then keep reading.
Every liquid has a tendency to minimize its surface area. So it behaves like a stretched elastic membrane of rubber. Now it is clear that there is a tension in the surface of a liquid and it is called surface tension.
Surface tension is a scaler quantity.
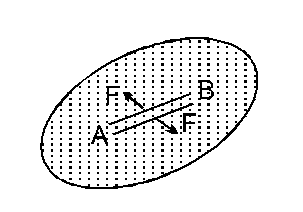 So, $\mathrm{T}=\frac{\mathrm{F}}{\ell}$ where T = surface tension of liquid.
So, $\mathrm{T}=\frac{\mathrm{F}}{\ell}$ where T = surface tension of liquid.
Unit of surface tension in MKS system
N/m and $\mathrm{J} / \mathrm{m}^{2}$
CGS system
Dyne/cm and $\mathrm{erg} / \mathrm{cm}^{2}$
Dimension of surface tension = $\mathrm{M}^{1} \mathrm{~L}^{0} \mathrm{~T}^{-2}$
Sol. The ring is in contact with water along its inner and outer circumference so when pulled out the total force on it due to surface tension will be
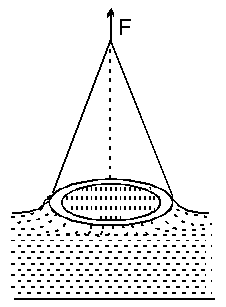
$\mathrm{F}=\mathrm{T}\left(2 \pi \mathrm{r}_{1}+2 \pi \mathrm{r}_{2}\right)$
So
$\mathrm{T}=\frac{\mathrm{mg}}{2 \pi\left(\mathrm{r}_{1}+\mathrm{r}_{2}\right)}$
[as F = mg]
i.e.
$\mathrm{T}=\frac{3.97 \times 980}{3.14 \times(8.5+8.7)}$
$=72.13$ dyne/cm
So, that's all from this topic. I hope you get a complete idea about what is Surface tension in physics? and example of surface tension. If you found this article helpful then please share it with your friends.
For a better understanding of this chapter, please check the detailed notes of the Mechanical Properties of fluids. If you want more Free Learning Videos and Study Material Then don't forget to download the eSaral App.
Surface Tension
Surface tension is the property of a liquid, which depends on the nature of the liquid.Every liquid has a tendency to minimize its surface area. So it behaves like a stretched elastic membrane of rubber. Now it is clear that there is a tension in the surface of a liquid and it is called surface tension.
Surface tension is a scaler quantity.
Definition of surface tension:
The surface tension of a liquid is defined as the force per unit length in the plane of the liquid surface at right angles to either side of an imaginary line drawn on that surface.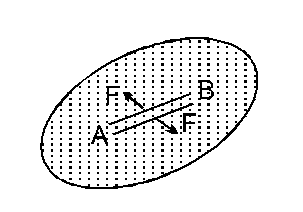 So, $\mathrm{T}=\frac{\mathrm{F}}{\ell}$ where T = surface tension of liquid.
So, $\mathrm{T}=\frac{\mathrm{F}}{\ell}$ where T = surface tension of liquid.
Unit of surface tension in MKS system
N/m and $\mathrm{J} / \mathrm{m}^{2}$
CGS system
Dyne/cm and $\mathrm{erg} / \mathrm{cm}^{2}$
Dimension of surface tension = $\mathrm{M}^{1} \mathrm{~L}^{0} \mathrm{~T}^{-2}$
Examples of surface tension :
- After putting a glass rod in a burner flame its end becomes spherical.
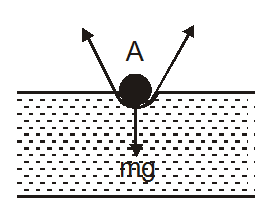
- Floating of a needle on water: If we put a needle on blotting paper and placed it on water surface then blotting paper will sink after getting wet and the needle remains floating on the water surface. The reason behind it is surface tension.Two forces acts on needle surface tension T and weight W of the needle after dividing surface tension force in horizontal and vertical components, horizontal components cancel each other and the vertical components adds which balances the weight of needle so needle floats on water surface
- Soap water cleans cloths well in comparison to impure water.
- Filling the ink in the nib of a pen.
- The dancing of camfor on the water surface.
Effects on surface tension:
- Effect of impurity: The surface tension of a liquid increases due to solvent impurity in the liquid. By solving salt in water, the surface tension of water increases. If the solvent impurity is less soluble then surface tension decreases example after solving soap in water the surface tension of decreases.
- Effect of temperature: Due to increase in temperature the cohesive forces between the molecules of the liquid so the surface tension decreases and on critical temperature it becomes zero.
- Effect of floating impurity: If there is dust, oil or grease then also surface tension of liquid decreases.
- Flowing of electricity in the liquid: Flowing of electricity in the liquid a force acts normally on the free surface of the liquid. Therefore, the surface tension of the liquid. decreases.
Sol. The ring is in contact with water along its inner and outer circumference so when pulled out the total force on it due to surface tension will be

$\mathrm{F}=\mathrm{T}\left(2 \pi \mathrm{r}_{1}+2 \pi \mathrm{r}_{2}\right)$
So
$\mathrm{T}=\frac{\mathrm{mg}}{2 \pi\left(\mathrm{r}_{1}+\mathrm{r}_{2}\right)}$
[as F = mg]
i.e.
$\mathrm{T}=\frac{3.97 \times 980}{3.14 \times(8.5+8.7)}$
$=72.13$ dyne/cm
So, that's all from this topic. I hope you get a complete idea about what is Surface tension in physics? and example of surface tension. If you found this article helpful then please share it with your friends.
For a better understanding of this chapter, please check the detailed notes of the Mechanical Properties of fluids. If you want more Free Learning Videos and Study Material Then don't forget to download the eSaral App.
