JEE Main Previous Year Papers Questions of Chemistry With Solutions are available at eSaral.
Simulator
Previous Years AIEEE/JEE Mains Questions
Q. The bond dissociation energy of B–F in $\mathrm{BF}_{3}$ is 646 kJ $\mathrm{mol}^{-1}$whereas that of C–F in $\mathrm{CF}_{4}$ is 515 kJ mol–1. The correct reason for higher B–F bond dissociation energy as compared to that of C–F is :-
(1) Significant $\mathrm{p} \pi-\mathrm{p} \pi$ interaction between B and F in $\mathrm{BF}_{3}$whereas there is not possibility of such interaction between C and F in $\mathrm{CF}_{4}$.
(2) Lower degree of p – p interaction between B and F in $\mathrm{BF}_{3}$ than that
between C and F in $\mathrm{CF}_{4}$
(3)Smaller size of B-atom as compared to that of C-atom
(4) Stronger bond between B and F in $\mathrm{BF}_{3}$ as compared to that between C and F in $\mathrm{CF}_{4}$
[AIEEE-2009]
Ans. (1)
Due to back bonding in BF3 molecule all B–F bond having partial double bond character.
Q. Using MO theory predict which of the following species has the shortest bond length ?
(1) $\mathrm{o}_{2}^{-}$
(2) $\mathrm{O}_{2}^{2-}$
(3) $\mathrm{O}_{2}^{2+}$
(4) $\mathrm{O}_{2}^{+}$
[AIEEE-2009]
Ans. (3)
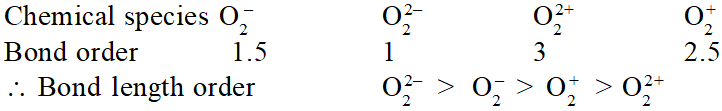
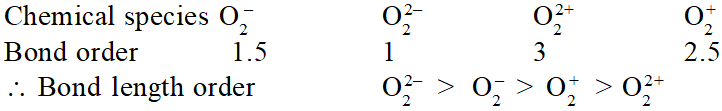
Q. The hybridisation of orbitals of N atom in $\mathrm{NO}_{3}^{-}, \mathrm{NO}_{2}^{+}$ and $\mathrm{NH}_{4}^{+}$ are respectively:-
[AIEEE-2011]
Ans. (4)
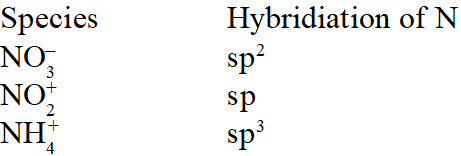
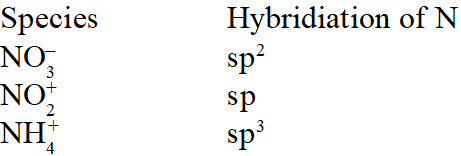
Q. The structure of $\mathrm{IF}_{7}$ is :-
(1) octahedral
(2) pentagonal bipyramid
(3) square pyramid
(4) trigonal bipyramid
[AIEEE-2011]
Ans. (2)
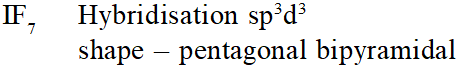
Q. Among the following the maximum covalent character is shown by the compound :-
(1) $\mathrm{AlCl}_{3}$
(2) $\mathrm{MgCl}_{2}$
(3) FeCl $_{2}$
(4) $\mathrm{SnCl}_{2}$
[AIEEE-2011]
Ans. (1)
$\mathrm{Al}^{+3}$ having highest polarizing power than other : having greater covalent character
Q. Which of the following has maximum number of lone pairs associated with Xe
(1) $\mathrm{XeO}_{3}$
(2) $\mathrm{XeF}_{4}$
(3) XeF $_{6}$
(4) $\mathrm{XeF}_{2}$
[AIEEE-2011]
Ans. (4)
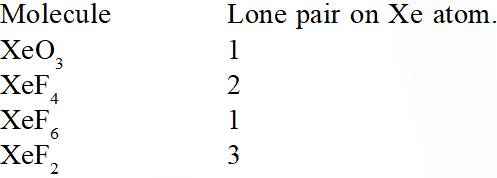
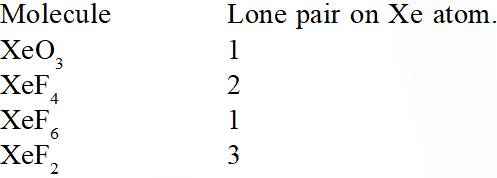
Q. The number of types of bonds between two carbon atoms in calcium carbide is :-
(1) One sigma, two pi
(2) One sigma, one pi
(3) Two sigma, one pi
(4) Two sigma, two pi
[AIEEE-2011]
Ans. (1)
$\mathrm{Ca}^{+2}(\mathrm{C} \equiv \mathrm{C})^{2-}$
Q. The molecule having smallest bond angle is :-
(1) $\mathrm{PCl}_{3}$
(2) $\mathrm{NCl}_{3}$
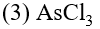 (4) $\mathrm{SbCl}_{3}$
[AIEEE-2012]
(4) $\mathrm{SbCl}_{3}$
[AIEEE-2012]
Ans. (4)
Bond angle order $\mathrm{NCl}_{3}>\mathrm{PCl}_{3}>\mathrm{AsCl}_{3}>\mathrm{SbCl}_{3}$
Q. In which of the following pairs the two species are not isostructural ?
(1) $\mathrm{AIF}_{6}^{3-}$ and $\mathrm{SF}_{6}$
(3) $\mathrm{CO}_{3}^{2-}$ and $\mathrm{NO}_{3}^{-}$
(3) $\mathrm{PCl}_{4}^{+}$ and $\mathrm{SiCl}_{4}$
(4) $\mathrm{PF}_{5}$ and $\mathrm{BrF}_{5}$
[AIEEE-2012]
Ans. (4)


Q. The number of S–S bonds in $\mathrm{SO}_{3}, \mathrm{S}_{2} \mathrm{O}_{3}^{2-}, \mathrm{S}_{2} \mathrm{O}_{6}^{2-}$ and $\mathrm{S}_{2} \mathrm{O}_{8}^{2-}$ respectively are :-
(1) 1, 0, 1, 0 (2) 0, 1, 1, 0 (3) 1, 0, 0, 1 (4) 0, 1, 0, 1
[JEE-MAINS(Online) 2012]
Ans. (2)
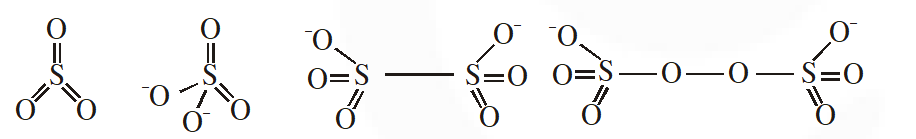
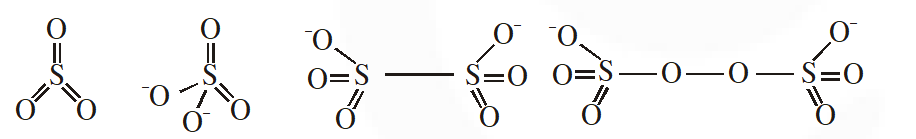
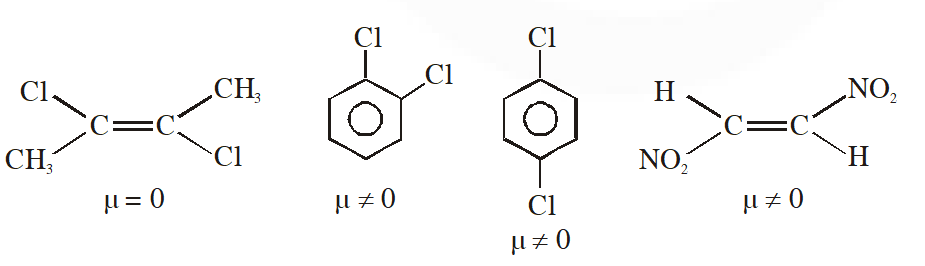
Q. Dipole moment is shown by :-
(1) trans-2, 3-dichloro- 2-butene
(2) 1, 2-dichlorobenzene
(3) 1, 4-dichlorobenzene
(4) trans-1, 2-dinitroethene
[JEE-MAINS(Online) 2012]
Ans. (2)

Q. Among the following species which two have trigonal bipyramidal shape ?
 [JEE-MAINS(Online) 2012]
[JEE-MAINS(Online) 2012]
Ans. (Bonus)
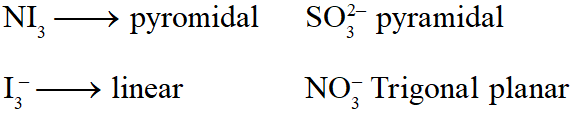
Q. Among the following, the species having the smallest bond is :-
(1) NO
(2) $\mathrm{NO}^{+}$
(3) $\mathrm{O}_{2}$
(4) $\mathrm{NO}^{-}$
[JEE-MAINS(Online) 2012]
Ans. (2)

Q. Based on lattice energy and other considerations, which one of the following alkali metal chloride is expected to have the highest melting point ?
(1) RbCl
(2) LiCl
(3) KCl
(4) NaCl
[JEE-MAINS(Online) 2012]
Ans. (4)
Highest melting is of NaCl
Q. Which of the following has the square planar structure :-
(1) $\mathrm{NH}_{4}^{+}$
(2) $\mathrm{CCl}_{4}$
(3) $\mathrm{XeF}_{4}$
(4) $\mathrm{BF}_{4}^{-}$
[JEE-MAINS(Online) 2012]
Ans. (3)
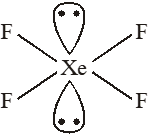 Hybridisation $\mathrm{Sp}^{3} \mathrm{d}^{2}$
Shape – square planar
Hybridisation $\mathrm{Sp}^{3} \mathrm{d}^{2}$
Shape – square planar
 Hybridisation $\mathrm{Sp}^{3} \mathrm{d}^{2}$
Shape – square planar
Hybridisation $\mathrm{Sp}^{3} \mathrm{d}^{2}$
Shape – square planar
Q. The compound of Xenon with zero dipole moment is :-
(1) $\mathrm{XeO}_{3}$
(2) $\mathrm{XeO}_{2}$
(3) $\mathrm{XeF}_{4}$
(4) $\mathrm{XeOF}_{4}$
[JEE-MAINS(Online) 2012]
Ans. (3)
Q. Among the following the molecule with the lowest dipole moment is :-
(1) $\mathrm{CHCl}_{3}$
(2) $\mathrm{CH}_{2} \mathrm{Cl}_{2}$
(3) $\mathrm{CCl}_{4}$
(4) $\mathrm{CH}_{3} \mathrm{Cl}$
[JEE-MAINS(Online) 2012]
Ans. (3)
$\mathrm{CCl}_{4}<\mathrm{CHCl}_{3}<\mathrm{CH}_{2} \mathrm{Cl}_{2}<\mathrm{CH}_{3} \mathrm{Cl}(\text { Dipolar moment })$
Q. The formation of molecular complex $\mathrm{BF}_{3}-\mathrm{NH}_{3}$ results in a change in hybridisation of boron
(1) from $\operatorname{sp}^{3}$ to $\mathrm{sp}^{3} \mathrm{d}$
(2) from $\operatorname{sp}^{2}$ to $\mathrm{dsp}^{2}$
(3) from $\operatorname{sp}^{3}$ to $\mathrm{sp}^{2}$
(4) from $\operatorname{sp}^{2}$ to $\mathrm{sp}^{3}$
[JEE-MAINS(Online) 2012]
Ans. (4)
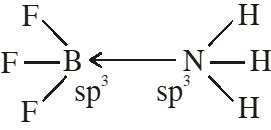
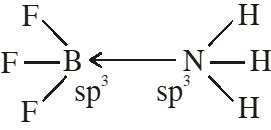
Q. Among the following chloro-compound having the lowest dipole moment is :-
(1) $\mathrm{CH}_{2} \mathrm{Cl}_{2}$
(2) $\mathrm{CH}_{3} \mathrm{Cl}$
(3) 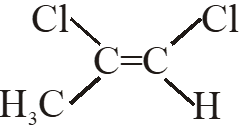 (4)
(4) 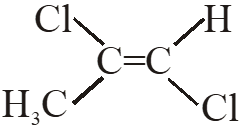
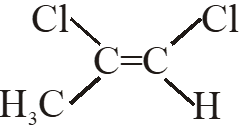 (4)
(4) 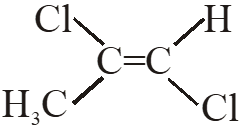
Ans. (1)
$\mathrm{CH}_{2} \mathrm{Cl}_{2}$ has lowest dipole moment
Q. Which one of the following molecules is expected to exhibit diamagnetic behaviour ?
(1) $\mathrm{C}_{2}$
(2) $\mathrm{N}_{2}$
(3) $\mathrm{O}_{2}$
(4) $\mathrm{S}_{2}$
[AIEEE-2013]
Ans. (2)
$\mathrm{N}_{2}$ is diamagnetic
Q. Which of the following is the wrong statement?
(1) ONCl and $\mathrm{ONO}^{-}$ are not isoelectronic
(2) $\mathrm{O}_{3}$ molecule is bent
(3) Ozone is violet-black in solid state
(4) Ozone is diamagnetic gas
[JEE-maIN 2013]
Ans. (1)
Number of electron in ONCl an $\mathrm{ONO}^{-}$ is 32 & 24 respectively.
Q. In which of the following pairs of molecules/ions, both the species are not likely to exist ?
(1) $\mathrm{H}_{2}^{+}, \mathrm{He}_{2}^{2-}$
(2) $\mathrm{H}_{2}^{-}, \mathrm{He}_{2}^{2-}$
(3) $\mathrm{H}_{2}^{2+}, \mathrm{He}_{2}$
(4) $\mathrm{H}_{2}^{-}, \mathrm{H} \mathrm{e}_{2}^{2+}$
[JEE-main 2013]
Ans. (3)
Bond order of $\mathrm{H}_{2}^{2+} \& \mathrm{He}_{2}$ is zero i.e. these molecule do not exist.
Q. Stability of the species $\mathrm{Li}_{2}, \quad \mathrm{Li}_{2}^{-}$ and $\mathrm{Li}_{2}^{+}$ increases in the order of :-
(1) $\mathrm{Li}_{2}<\mathrm{Li}_{2}^{+}<\mathrm{Li}_{2}^{-}$
(2) $\mathrm{Li}_{2}^{-}<\mathrm{Li}_{2}^{+}<\mathrm{Li}_{2}$
(3) $\mathrm{Li}_{2}<\mathrm{Li}_{2}<\mathrm{Li}_{2}^{+}$
(4) $\mathrm{Li}_{2}^{-}<\mathrm{Li}_{2}<\mathrm{Li}_{2}^{+}$
[JEE-main 2013]
Ans. (2)
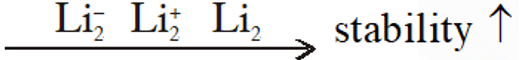
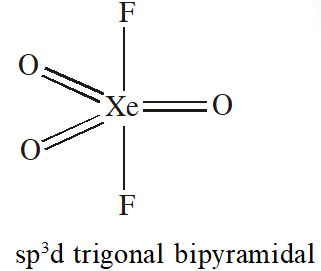
Q. Trigonal bipyramidal geometry is shown by:
(1) $\mathrm{XeO}_{3} \mathrm{F}_{2}$
(2) $\mathrm{XeOF}_{2}$
(3) $\left[\mathrm{XeF}_{8}\right]^{2-}$
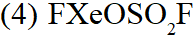 [JEE-MAINS(Online) 2013]
[JEE-MAINS(Online) 2013]
Ans. (1)

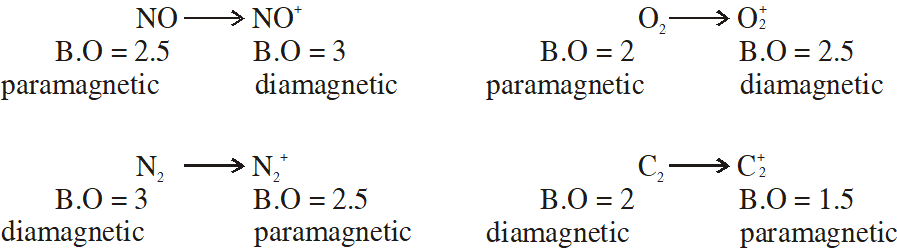
Q. In which of the following ionization processes the bond energy has increased and also the magnetic behaviour has changed from paramagnetic to diamagnetic ?
(1) $\mathrm{NO} \rightarrow \mathrm{NO}^{+}$
(2) $\mathrm{O}_{2} \rightarrow \mathrm{O}_{2}^{+}$
(3) $\mathrm{N}_{2} \rightarrow \mathrm{N}_{2}^{+}$
( 4) $\mathrm{C}_{2} \rightarrow \mathrm{C}_{2}^{+}$
[JEE-MAINS(Online) 2013]
Ans. (1)

Q. Which one of the following molecules is polar?
(1) $\mathrm{CF}_{4}$
(2) $\mathrm{SbF}_{5}$
(3) $\mathrm{IF}_{5}$
(4) $\mathrm{XeF}_{4}$
[JEE-MAINS(Online) 2013]
Ans. (3)
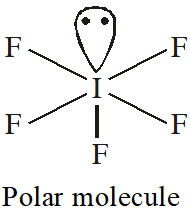
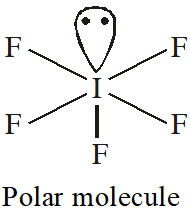
Q. Oxidation state of sulphur in anions $\mathrm{SO}_{3,}^{2-} \mathrm{S}_{2} \mathrm{O}_{4}^{2-}$ and $\mathrm{S}_{2} \mathrm{O}_{6}^{2-}$ increases in the orders :
(1) $\mathrm{S}_{2} \mathrm{O}_{6}^{2-}<\mathrm{S}_{2} \mathrm{O}_{4}^{2-}<\mathrm{SO}_{3}^{2-}$
(2) $\mathrm{SO}_{3}^{2-}<\mathrm{S}_{2} \mathrm{O}_{4}^{2-}<\mathrm{S}_{2} \mathrm{O}_{6}^{2-}$
(3) $\mathrm{S}_{2} \mathrm{O}_{4}^{2-}<\mathrm{SO}_{3}^{2-}<\mathrm{S}_{2} \mathrm{O}_{6}^{2-}$
(4) $\mathrm{S}_{2} \mathrm{O}_{4}^{2-}<\mathrm{S}_{2} \mathrm{O}_{6}^{2-}<\mathrm{SO}_{3}^{2-}$
[JEE-MAINS(Online) 2013]
Ans. (3)
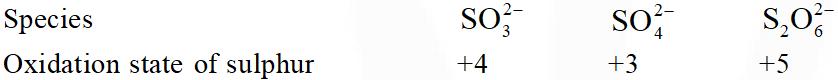
Q. Bond order normally gives idea of stability of a molecular species. All the molecules viz. $\mathrm{H}_{2}, \mathrm{Li}_{2}$ and $\mathrm{B}_{2}$ have the same bond order yet they are not equally stable. Their stability order is:
(1) $\mathrm{Li}_{2}>\mathrm{H}_{2}>\mathrm{B}_{2}$
(2) $\mathrm{H}_{2}>\mathrm{B}_{2}>\mathrm{Li}_{2}$
(3) $\mathrm{B}_{2}>\mathrm{H}_{2}>\mathrm{Li}_{2}$
(4) $\mathrm{Li}_{2}>\mathrm{B}_{2}>\mathrm{H}_{2}$
[JEE-MAINS(Online) 2013]
Ans. (2)
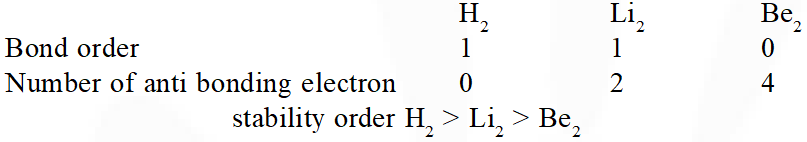
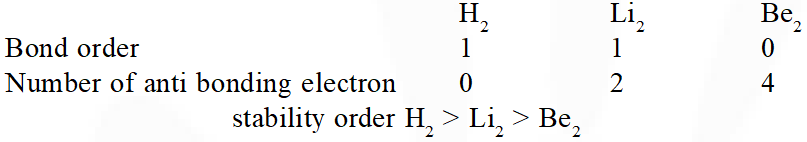
Q. The solubility order for alkali metal fluoride in water is :
(1) $\mathrm{LiF}<\mathrm{NaF}<\mathrm{KF}<\mathrm{RbF}$
(2) $\mathrm{LiF}>\mathrm{NaF}>\mathrm{KF}>\mathrm{RbF}$
(3) $\mathrm{RbF}<\mathrm{KF}<\mathrm{NaF}<\mathrm{LiF}$
(4) $\mathrm{LiF}<\mathrm{RbF}<\mathrm{KF}<\mathrm{NaF}$
[JEE-MAINS(Online) 2013]
Ans. (1)
Solubility order
LiF < NaF < KF < RbF
Q. $\mathrm{XeO}_{4}$ molecule is tetrahedral having :
(1) Two p $\pi$ -d $\pi$ bonds
(2) Four p $\pi$ - d $\pi$ bonds
(3) One $\mathrm{p} \pi-\mathrm{d} \pi$ bond
(4) Three p $\pi$ -d $\pi$ bonds
[JEE-MAINS(Online) 2013]
Ans. (2)
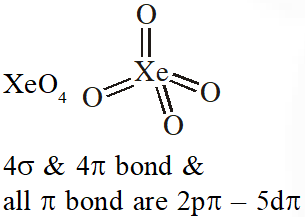
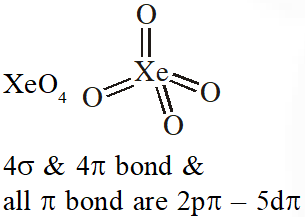
Q. Bond distance in HF is 9.17 × $10^{-11}$ m. Dipole moment of HF is 6.104 × 10–30 Cm.
The percent ionic character in HF will be : (electron charge = 1.60 × $10^{-19}$ C)
(1) 61.0%
(2) 38.0%
(3) 35.5%
(4) 41.5%
[JEE-MAINS(Online) 2013]
Ans. (4)
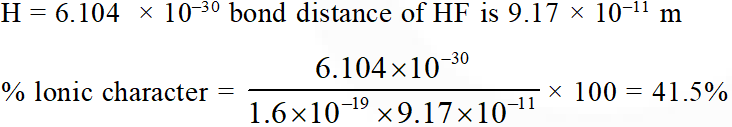
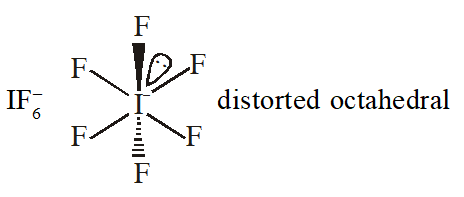
Q. The shape of $\mathrm{IF}_{6}^{-}$ is :
(1) Trigonally distorted octahedron
(2) Pyramidal
(3) Octahedral
(4) Square antiprism
[JEE-MAINS(Online) 2013]
Ans. (1)

Q. Which has trigonal bipyramidal shape ?
(1) $\mathrm{XeOF}_{4}$
(2) $\mathrm{XeO}_{3}$
(3) $\mathrm{XeO}_{3} \mathrm{F}_{2}$
(4) $\mathrm{XeOF}_{2}$
[JEE-MAINS(Online) 2013]
Ans. (3)
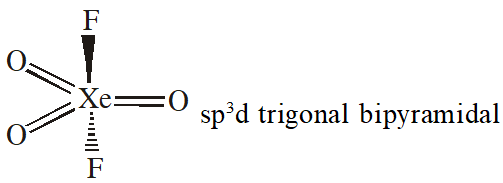
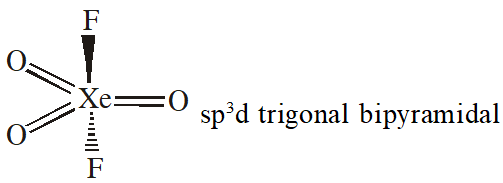
Q. The catenation tendency of C, Si and Ge is in the order Ge < Si < C. The bond energies
(in kJ $\mathrm{mol}^{-1}$) of C — C, Si —Si and Ge—Ge bonds are respectively :
(1) 348, 260, 297
(2) 348, 297, 260
(3) 297, 348, 260
(4) 260, 297, 348
[JEE-MAINS(Online) 2013]
Ans. (2)
Catenation/Bond energy order
C – C > Si – Si > Ge – Ge
Q. In which of the following sets, all the given species are isostructural ?
(1) $\mathrm{BF}_{3}, \mathrm{NF}_{3}, \mathrm{PF}_{3}, \mathrm{AlF}_{3}$
(2) $\mathrm{PCl}_{3}, \mathrm{AlCl}_{3}, \mathrm{BCl}_{3}, \mathrm{SbCl}_{3}$
(3) $\mathrm{BF}_{4}^{-}, \mathrm{CCl}_{4}, \mathrm{NH}_{4}^{+}, \mathrm{PCl}_{4}^{+}$
(4) $\mathrm{CO}_{2}, \mathrm{NO}_{2}, \mathrm{ClO}_{2}, \mathrm{SiO}_{2}$
[JEE-MAINS(Online) 2013]
Ans. (3)
$\mathrm{BF}_{4}^{-}, \mathrm{CC}_{4}, \mathrm{NH}_{4}^{+}, \mathrm{PCl}_{4}^{+}$ are terahedral
Q. The internuclear distances in O —O bonds for O, $\mathrm{O}_{2}, \mathrm{O}^{-}$2 and O respectively are :
(1) 1.49 Å, 1.21 Å, 1.12 Å, 1.30 Å
(2) 1.30 Å, 1.49 Å, 1.12 Å, 1.21
(3) 1.12 Å, 1.21 Å, 1.30 Å, 1.49 Å
(4) 1.21 Å, 1.12 Å, 1.49 Å, 1.30 Å
[JEE-MAINS(Online) 2013]
Ans. (3)
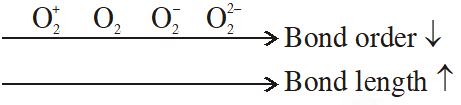
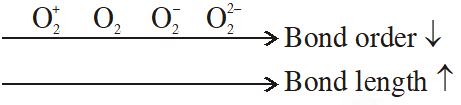
Q. Which one of the following properties is not shown by NO ?
(1) It combines with oxygen to form nitrogen dioxide
(2) It's bond order is 2.5
(3) It is diamagnetic in gaseous state
(4) It is a neutral oxide
[JEE-main 2014]
Ans. (3)


Q. For which of the following molecule significant $\mu \neq 0$
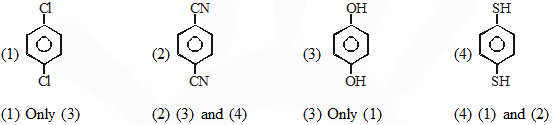 [JEE-main 2014]
[JEE-main 2014]
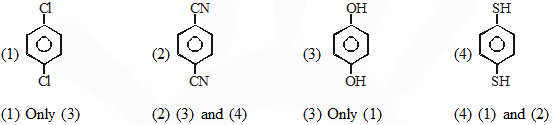 [JEE-main 2014]
[JEE-main 2014]
Ans. (2)
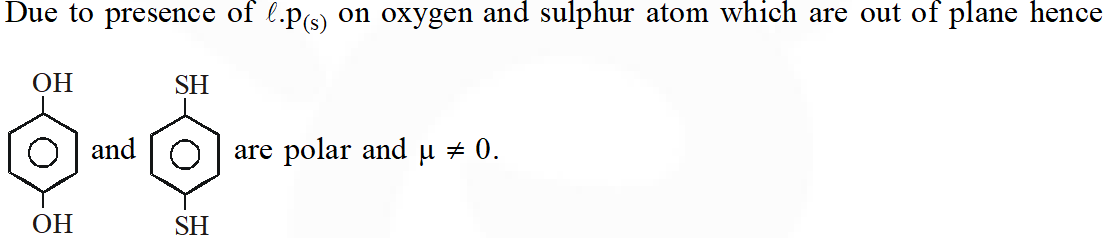
Q. The number and type of bonds in $\mathrm{C}_{2}^{2-} \mathrm{ion}$ in $\mathrm{CaC}_{2}$ are:
(1) Two $\sigma$ bonds and one $\pi-$ bond
(2) Two $\sigma$ bonds and two $\pi-$ bonds
(3) One $\sigma$ bond and two $\pi-$ bonds
(4) One $\sigma$ bond and one $\pi-$ bond
[JEE-MAINS(Online) 2014]
Ans. (3)
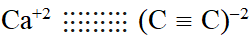
Q. For the compounds $\mathrm{CH}_{3} \mathrm{Cl}, \mathrm{CH}_{3} \mathrm{Br}, \mathrm{CH}_{3} \mathrm{I}$ and $\mathrm{CH}_{3} \mathrm{F}$
(1) $\mathrm{CH}_{3} \mathrm{F}<\mathrm{CH}_{3} \mathrm{Br}<\mathrm{CH}_{3} \mathrm{Cl}<\mathrm{CH}_{3} \mathrm{I}$
(2) $\mathrm{CH}_{3} \mathrm{F}<\mathrm{CH}_{3} \mathrm{Cl}<\mathrm{CH}_{3} \mathrm{Br}<\mathrm{CH}_{3} \mathrm{I}$
(3) $\mathrm{CH}_{3} \mathrm{Cl}<\mathrm{CH}_{3} \mathrm{Br}<\mathrm{CH}_{3} \mathrm{F}<\mathrm{CH}_{3} \mathrm{I}$, the correct order of increasing C-halogen bond length is :
(4) $\mathrm{CH}_{3} \mathrm{F}<\mathrm{CH}_{3} \mathrm{I}<\mathrm{CH}_{3} \mathrm{Br}<\mathrm{CH}_{3} \mathrm{Cl}$
[JEE-MAINS(Online) 2014]
Ans. (2)

Q. Which of the following has unpaired electron(s) ?
(1) $\mathrm{O}_{2}^{-}$
(2) $\mathrm{N}_{2}^{2+}$
(3) $\mathrm{O}_{2}^{2-}$
(4) $\mathrm{N}_{2}$
[JEE-MAINS(Online) 2014]
Ans. (1)
$\mathrm{O}_{2}^{-}$ ha one unpaired electron
Q. In allene $\left(\mathrm{C}_{3} \mathrm{H}_{4}\right)$, the type(s) of hybridization of the carbon atoms is (are):
(1) only sp $^{2}$
(2) $\mathrm{sp}^{2}$ and $\mathrm{sp}$
(3) sp and sp $^{3}$
(4) $\mathrm{sp}^{2}$ and $\mathrm{sp}^{3}$
[JEE-MAINS(Online) 2014]
Ans. (2)
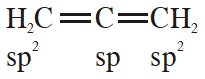
Q. Shapes of certain interhalogen compounds are stated below. Which one of them is not correctly stated?
(1) IF $_{7}:$ Pentagonal bipyramid
(2) BrF $_{5}:$ Trigonal bipyramid
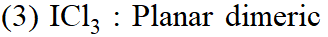 (4) $\mathrm{BrF}_{3}:$ Planar T-shaped
[JEE-MAINS(Online) 2014]
(4) $\mathrm{BrF}_{3}:$ Planar T-shaped
[JEE-MAINS(Online) 2014]
Ans. (2)
$\mathrm{BrF}_{5}$ have square pyramidal shape.
Q. The correct order of bond dissociation energy among $\mathrm{N}_{2}, \mathrm{O}_{2}, \mathrm{o}_{2}^{-}$ is shown in which of the following arrangements?
(1) $\mathrm{N}_{2}>\mathrm{O}_{2}>\mathrm{O}_{2}^{-}$
(2) $\mathrm{O}_{2}>\mathrm{O}_{2}^{-}>\mathrm{N}_{2}$
(3) $\mathrm{N}_{2}>\mathrm{O}_{2}^{-}>\mathrm{O}_{2}$
(4) $\mathrm{O}_{2}^{-}>\mathrm{O}_{2}>\mathrm{N}_{2}$
[JEE-MAINS(Online) 2014]
Ans. (1)
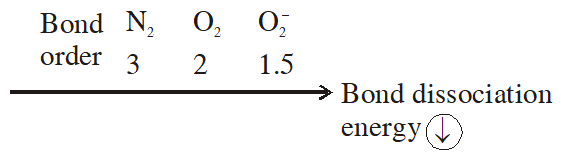
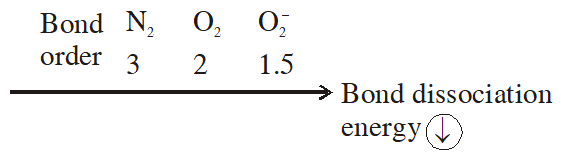
Q. Which of the following molecules has two sigma$(\sigma)$ and two $\operatorname{pi}(\pi)$ bonds :-
(1) HCN
(2) $\mathrm{C}_{2} \mathrm{H}_{2} \mathrm{Cl}_{2}$
(3) $\mathrm{N}_{2} \mathrm{F}_{2}$
(4) $\mathrm{C}_{2} \mathrm{H}_{4}$
[JEE-MAINS(Online) 2014]
Ans. (1)
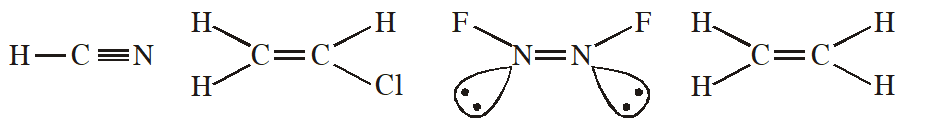
Q. Which one of the following molecules is paramagnetic?
(1) NO
( 2) $\mathrm{O}_{3}$
(3) $\mathrm{N}_{2}$
(4) CO
[JEE-MAINS(Online) 2014]
Ans. (1)
NO has unpaired e $^{-} \therefore$ paramagnetic is nature
Q. Amongst LiCl, RbCl, $\mathrm{BeCl}_{2}$ and $\mathrm{MgCl}_{2}$ the compounds with the greatest and the least ionic character, respectively are :
(1) $\mathrm{RbCl}$ and $\mathrm{MgCl}_{2}$
(2) LiCl and RbCl
(3) $\mathrm{MgCl}_{2}$ and $\mathrm{BeCl}_{2}$
(4) $\mathrm{RbCl}$ and $\mathrm{BeCl}_{2}$
[JEE-MAINS(Online) 2014]
Ans. (4)
RbCl has highest ionic & and $\mathrm{BeCl}_{2}$ is most covalent
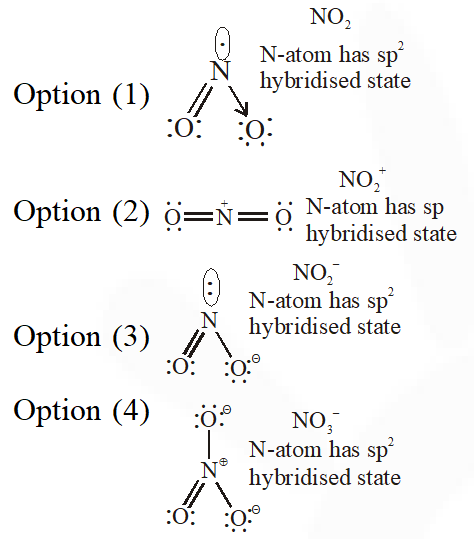
Q. The species in which the N atom is in a state of sp hybridization is :-
(1) $\mathrm{NO}_{2}$
(2) $\mathrm{NO}_{2}^{+}$
(3) $\mathrm{NO}_{2}^{-}$
(4) $\mathrm{NO}_{3}^{-}$
[JEE-MAINS 2016]
Ans. (2)

Q. Which of the following species is not paramagnetic :-
(1)NO
(2) CO
(3) $\mathrm{O}_{2}$
(4) $\mathrm{B}_{2}$
[JEE-MAINS 2017]
Ans. (2)


Q. Total number of lone pair of electrons in $\mathrm{I}_{3}^{-} \mathrm{ion}$ is
(1) 6 (2) 9 (3) 12 (4) 3
[JEE-MAINS 2018]
Ans. (2)
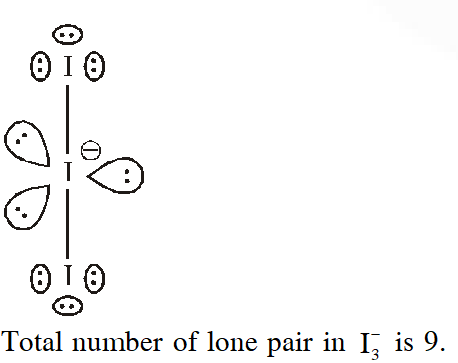
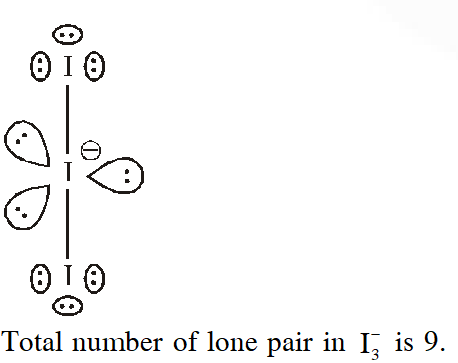
Q. According to molecular orbital theory, which of the following will not be a viable molecule ?
(1) $\mathrm{He}_{2}^{+}$
(2) $\mathrm{H}_{2}^{-}$
(3) $\mathrm{H}_{2}^{2-}$
(4) $\mathrm{He}_{2}^{2+}$
[JEE-MAINS 2018]
Ans. (3)
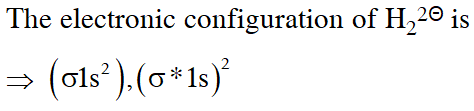
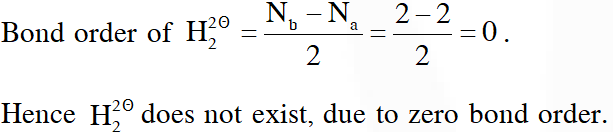
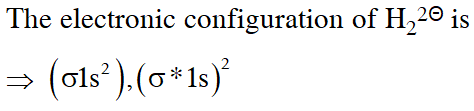
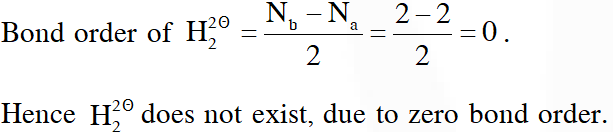
Q. Which of the following compounds contain(s) no covalent bond(s) ?
$\mathrm{KCl}, \mathrm{PH}_{3}, \mathrm{O}_{2}, \mathrm{B}_{2} \mathrm{H}_{6}, \mathrm{H}_{2} \mathrm{SO}_{4}$
$(1) \mathrm{KCl}, \mathrm{H}_{2} \mathrm{SO}_{4}$
(2) KC1
(3) $\mathrm{KCl}, \mathrm{B}_{2} \mathrm{H}_{6}$
(4) $\mathrm{KCl}, \mathrm{B}_{2} \mathrm{H}_{6}, \mathrm{PH}_{3}$
[JEE-MAINS 2018]
Ans. (2)
Comments
Brandy
Feb. 11, 2024, 1:14 a.m.
Wow, wonderful blog format! How long have you ever been blogging
for? you made blogging look easy. The total glance of your website is great, as neatly as the content!
You can see similar: Crystallon.top and here Crystallon.top
Alakh Pandey ( Physics wallah)
April 29, 2021, 6:24 p.m.
Are madharchodo randio ke aulaad gaaaaaaaaaaaaar far ke bajaaaaaaaar ke le leeeengeeee
Karthik
Feb. 18, 2021, 5:05 p.m.
Chemical bonding is so difficult to remember structures
And I want some structure
P shree mohan chandra naik
Feb. 16, 2021, 3:41 p.m.
it saved my time a lot thank you so much it really helped me a lot
