
NCERT Solutions for Class 12 Chemistry Chapter 4 Chemical Kinetics PDF
Hey, are you a class 12 student and looking for ways to download NCERT Solutions for Class 12 Chemistry Chapter 4 Chemical Kinetics PDF? If yes. Then read this post till the end.In this article, we have listed NCERT Solutions for Class 12 Chemistry Chapter 4 Chemical Kinetics in PDF that are prepared by Kota’s top IITian Faculties by keeping Simplicity in mind.
If you want to learn and understand class 12 Chemistry Chapter 4 "Chemical Kinetics" in an easy way then you can use these solutions PDF.
NCERT Solutions helps students to Practice important concepts of subjects easily. Class 12 Chemistry solutions provide detailed explanations of all the NCERT questions that students can use to clear their doubts instantly.
If you want to score high in your class 12 Chemistry Exam then it is very important for you to have a good knowledge of all the important topics, so to learn and practice those topics you can use eSaral NCERT Solutions.
In this article, we have listed NCERT Solutions for Class 12 Chemistry Chapter 4 Chemical Kinetics PDF that you can download to start your preparations anytime.
So, without wasting more time Let’s start.
Download NCERT Solutions for Class 12 Chemistry Chapter 4 Chemical Kinetics PDF
Question 1. For the reaction $\mathrm{R} \rightarrow \mathrm{P}$, the concentration of a reactant changes from $0.03 \mathrm{M}$ to $0.02 \mathrm{M}$ in 25 minutes. Calculate the average rate of reaction using units of time both in minutes and seconds.
Solution. Given $[\mathrm{R}]_{1}=0,03 ;[\mathrm{R}]_{2}=0.02 ;$ Average rate of reaction $=$
$\frac{\text { change in concentration }}{\text { time intervel }}=\frac{\Delta[\mathrm{R}]}{\Delta \mathrm{t}}$
$=\frac{[R]_{2}-[R]_{1}}{t_{2}-t_{1}}$
Average rate of reaction in minutes $=-\frac{(0.02-0.03)}{25} \mathrm{M} \mathrm{min}^{-1}$
Negative sign indicate the decrease the concentration
$=-\frac{(-0.01)}{25} \mathrm{M} \min ^{-1}=4 \times 10^{-4} \mathrm{M} \mathrm{min}^{-1}$
Average rate of reaction in seconds $=\frac{4 \times 10^{-4}}{60} \mathrm{M} \mathrm{s}^{-1}$
$=6.67 \times 10^{-6} \mathrm{M} \mathrm{s}^{-1}$
Concept Insight:
Average rate depends upon the change in concentration of reactants or products and time taken for that change to occur.
Rate of disappearance of $\mathrm{R}=$ Decrease in concentration of $\mathrm{R} /$ Time taken
Rate of appearance of $\mathrm{P}=$ Increase in concentration of $\mathrm{P} /$ Time taken
Question 2. In a reaction, $2 \mathrm{~A} \rightarrow$ Products, the concentration of A decreases from $0.5 \mathrm{~mol} \mathrm{~L}^{-1}$ to $0.4 \mathrm{~mol} \mathrm{~L}^{-1}$ in 10 minutes. Calculate the rate during this interval?
Solution. Average rate $=\frac{1}{2} \frac{\Delta[\mathrm{A}]}{\Delta \mathrm{t}}$
$=-\frac{1}{2} \frac{[\mathrm{A}]_{2}-[\mathrm{A}]_{1}}{\mathrm{t}_{2}-\mathrm{t}_{1}}$
$=-\frac{1(0.4-0.5)}{2} \frac{(0.4-0.5)}{10}$
$=-\frac{1}{2} \frac{(-0.1)}{10}$
$=0.005 \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~min}^{-1}$
$=5 \times 10^{-3} \mathrm{M} \min ^{-1}$
Concept Insight:
For expression the rate of reaction where stoichiometric coefficients of reactants or products are not equal to one, rate of disappearance of any of the reactants or rate of appearance of products is divided by their respective stoichiometric coefficients.
Question 3. For a reaction, $\mathrm{A}+\mathrm{B} \rightarrow$ Product; the rate law is given by, $\mathrm{r}=\mathrm{k}[\mathrm{A}]^{\frac{1}{2}}[\mathrm{~B}]^{2}$. What is the order of the reaction?
Solution. Sum of power of the concentration of the reactants in the rate law expression is called the order of that chemical reaction.
Given $\mathrm{r}=\mathrm{k}[\mathrm{A}]^{\frac{1}{2}}[\mathrm{~B}]^{2}$
Hence the order of the reaction $=\frac{1}{2}+2=2 \frac{1}{2}=2.5$
Concept Insight: Sum of power of the concentration of the actants in the rate law expression is called the order of the chemical reaction.
Question 4. The conversion of molecules $X$ to $Y$ follows second order kinetics. If concentration of $X$ is increased to three times how will it affect the rate of formation of $\mathrm{Y}$?
Solution. The reaction $X \rightarrow Y$ follows second order kinetics.
Therefore, the rate equation for this reaction will be:
Rate $=k[X]^{2}$
Let $[\mathrm{X}]=\mathrm{a} \mathrm{mol} \mathrm{L}^{-1}$,
then equation (1) can be written as:
Rate $_{1}=\mathrm{K} .(\mathrm{a})^{2}$
$=\mathrm{ka}^{2}$
If the concentration of $X$ is increased to three times, then $[X]=3 a \mathrm{~mol} \mathrm{~L}^{-1}$
Now, the rate equation will be:
Rate $_{2}=\mathrm{k}(3 \mathrm{a})^{2}=9\left(\mathrm{ka}^{2}\right)$
Rate $_{2}=9$ Rate $_{1}$
Hence, the rate of formation will increase by nine times.
Question 5. A first-order reaction has a rate constant $1.1510^{-3} \mathrm{~s}^{-1}$. How long will $5 \mathrm{~g}$ of this reactant take to reduce to $3 \mathrm{~g}$ ?
Solution. Given:
Initial amount $[\mathrm{R}]_{0}=5 \mathrm{~g}$
Final concentration $[\mathrm{R}]=3 \mathrm{~g}$
Rate constant $(\mathrm{k})=1.15 \times 10^{-3} \mathrm{~s}^{-1}$
Formula:
$\mathrm{t}=\frac{2.303}{\mathrm{k}} \log \frac{[\mathrm{R}]_{0}}{[\mathrm{R}]}$
Put the values of $[R]_{0},[R]$, Rate constant $(\mathrm{k})$ in above formula
$\mathrm{t}=\frac{2.303}{1.15 \times 10^{-3}} \log \frac{5}{3}$ second
$=\frac{2.303}{1.15 \times 10^{-3}} \times 0.2219$ seconds
$=444.196$ second
$=444$ seconds (approx)
Question 6. Time required to decompose $\mathrm{SO}_{2} \mathrm{Cl}_{2}$ to half of its initial amount is 60 minutes. If the decomposition is a first order reaction, calculate the rate constant of the reaction.
Solution. Given: Time required to decompose $\mathrm{SO}_{2} \mathrm{Cl}_{2}$ to half of its initial amount $\left(\mathrm{t}_{1 / 2}\right)=$ 60 minutes
Formula for $t_{1 / 2}$ of $1^{\text {st }}$ order reaction,
$\mathrm{t}_{1 / 2}=\frac{0.693}{\mathrm{k}}$
$\therefore \mathrm{k}=\frac{0.693}{\mathrm{t}_{1 / 2}}$
By putting the value $t_{1 / 2}$ in the equation the $k=\frac{0.693}{60}$
$\mathrm{k}=0.01155 \mathrm{~min}^{-1}$
$\mathrm{k}=0.01155 / 60 \mathrm{sec}^{-1}=1.925 \times 10^{-4} \mathrm{~s}^{-1}$
Question 7. What will be the effect of temperature on rate constant?
Solution. The rate constant of reaction is doubled or triple with an $10^{\circ} \mathrm{C}$ or $\mathrm{K}$ rise in temperature.
However, the exact $\mathrm{d}$ rate of a chemical reaction on temperature is given by Arrhenius equation,
$\mathrm{k}=\mathrm{Ae}^{-\frac{\mathrm{Ea}}{\mathrm{RT}}}$
Where,
k = rate constant
A = Arrhenius factor or the frequency factor
T = temperature
R = gas constant
$\mathrm{E}_{\mathrm{a}}=$ activation energy
Concept Insight: The effect of temperature on rate constant is given by Arrhenius equation.
Question 8. The rate of the chemical reaction doubles for an increase of $10 \mathrm{~K}$ in absolute temperature from $298 \mathrm{~K}$. Calculate $\mathrm{E}_{\mathrm{a}}$.
Solution. Given that
initial temperature $\left(\mathrm{T}_{1}\right)=298 \mathrm{~K}$
final temperature $\left(\mathrm{T}_{2}\right)=(298+10) \mathrm{K}=308 \mathrm{~K}$
We know that the rate of the reaction doubles when temperature is increased by $10^{\circ}$
Therefore, let assume value of $\mathrm{k}_{1}=\mathrm{k}$ and that of $\mathrm{k}_{2}=2 \mathrm{k}$
Also, $\mathrm{R}=8.314 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}$
Formula: $\ln \frac{\mathrm{k}_{2}}{\mathrm{k}_{1}}=\frac{\mathrm{E}}{\mathrm{R}}\left[\frac{\mathrm{T}_{2}-\mathrm{T}_{1}}{\mathrm{~T}_{1} \mathrm{~T}_{2}}\right]$
$\log \frac{\mathrm{k}_{2}}{\mathrm{k}_{1}}=\frac{\mathrm{E}}{2.303 \mathrm{R}}\left[\frac{\mathrm{T}_{2}-\mathrm{T}_{1}}{\mathrm{~T}_{1} \mathrm{~T}_{2}}\right]$
$\log \frac{2 \mathrm{k}}{\mathrm{k}}=\frac{\mathrm{E}}{2.303 \mathrm{R}}\left[\frac{308-298}{298 \times 308}\right]$
We get:
$\log \frac{2 \mathrm{k}}{\mathrm{k}}=\frac{\mathrm{E}_{\mathrm{a}}}{2.303 \times 8.314}\left[\frac{10}{298 \times 308}\right]$
$\Rightarrow \log 2=\frac{\mathrm{E}_{\mathrm{a}}}{2.303 \times 8.314}\left[\frac{10}{298 \times 308}\right]$
$\Rightarrow \mathrm{E}_{\mathrm{a}}=\frac{2.303 \times 8.314 \times 298 \times 308 \times \log 2}{10}$
$=52897.78 \mathrm{Jmol}^{-1}$
$=52.9 \mathrm{k} \mathrm{J} \mathrm{mol}^{-1}$
Concept insight: The rate of reaction doubles with $10^{\circ}$ rise of temperature.
Question 9. The activation energy for the reaction $2 \mathrm{HI}(\mathrm{g}) \rightarrow \mathrm{H}_{2}(\mathrm{~g})+\mathrm{I}_{2}(\mathrm{~g})$ is $209.6 \mathrm{~kJ} \mathrm{~mol}^{-1}$ of $581 \mathrm{~K}$. Calculate the fraction of molecules of reactants having energy equal to or greater than activation energy?
Solution. Given that
Activation energy $\left.\left(\mathrm{E}_{\mathrm{a}}\right)=209.6 \mathrm{k}\right] \mathrm{mol}^{-1}$
$1 \mathrm{kj}=10000 \mathrm{j}$ $\mathrm{E}_{\mathrm{a}}=209500 \mathrm{~J} \mathrm{~mol}^{-1}$
Temperature $(\mathrm{T})=581 \mathrm{~K}$
Gas constant $(\mathrm{R})=8.314 \mathrm{JK}^{-1} \mathrm{~mol}^{-1}$
According to Arrhenius equation
$\mathrm{K}=\mathrm{A} \mathrm{e}^{-\mathrm{E}_{\mathrm{a}} / \mathrm{RT}}$ In this formula term $\mathrm{e}^{-\mathrm{E}_{\mathrm{a}} / \mathrm{RT}}$ represent the number of molecules which have energy equal or more than activation energy
Number of molecules $=\mathrm{e}^{-\mathrm{E}_{\mathrm{a}} / \mathrm{RT}}$
put the values we get number of molecules
Number of molecules $(\mathrm{x})=\mathrm{e}^{-\mathrm{E}_{\mathrm{a}} / \mathrm{RT}}$
$\Rightarrow \ln \mathrm{x}=-\mathrm{E}_{\mathrm{a}} / \mathrm{RT}$
$\Rightarrow \log \mathrm{x}=\frac{209500 \mathrm{~J} \mathrm{~mol}^{-1}}{2.303 \times 8.314 \mathrm{JK}^{-1} \mathrm{~mol}^{-1} \times 581}=18.8323$
Now, $x=$ Anti log $(18.8323)$
$=$ Anti $\log \overline{19} .1677$
$=1.471 \times 10^{-19}$
Back of chapter questions
Question 1. From the rate expression for the following reactions, determine their order of reaction and the dimensions of the rate constants.
(i) $3 \mathrm{NO}(\mathrm{g}) \rightarrow \mathrm{N}_{2} \mathrm{O}(\mathrm{g}) ;$ Rate $=\mathrm{k}[\mathrm{NO}]^{2}$
(ii) $\mathrm{H}_{2} \mathrm{O}_{2}(\mathrm{aq})+3 \mathrm{I}^{-}$(aq) $+2 \mathrm{H}^{+} \rightarrow 2 \mathrm{H}_{2} \mathrm{O}(\mathrm{I})$
$+\mathrm{I}_{3}^{-} ;$Rate $=\mathrm{l}\left[\mathrm{H}_{2} \mathrm{O}\right]\left[\mathrm{I}^{-}\right]$
(iii) $\mathrm{CH}_{3} \mathrm{CHO}(\mathrm{g}) \rightarrow \mathrm{CH}_{4}(\mathrm{~g})+\mathrm{CO}(\mathrm{g}) ;$ Rate $=\mathrm{k}\left[\mathrm{CH}_{2} \mathrm{CH} 0\right]^{\frac{3}{2}}$
(iv) $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{Cl}(\mathrm{g}) \rightarrow \mathrm{C}_{2} \mathrm{H}_{4}(\mathrm{~g})+\mathrm{HCl}(\mathrm{g}) ;$ Rate $=\mathrm{k}\left[\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{Cl}\right]$
Solution. Formula used for Dimension of $\mathrm{k}=\frac{\text { Rate }}{\text { [concentration] order of reaction }}=$
Rate $=$ mole/litre/time
(i) Give rate $=\mathrm{k}[\mathrm{NO}]^{2}$
Therefore, order of the reaction $=2$
Dimension of $\mathrm{k}=\frac{\text { Rate }}{[\mathrm{NO}]^{2}}$
$=\frac{\mathrm{mol} \mathrm{L}^{-1} \mathrm{~s}^{-1}}{\left(\mathrm{~mol} \mathrm{~L}^{-1}\right)^{2}}$
$=\frac{\mathrm{mol} \mathrm{L}^{-1} \mathrm{~s}^{-1}}{\mathrm{~mol}^{2} \mathrm{~L}^{-2}}$
$=\mathrm{L} \mathrm{mol}^{-1} \mathrm{~s}^{-1}$
(ii) Given rate $=\mathrm{k}\left[\mathrm{H}_{2} \mathrm{O}_{2}\right]\left[\mathrm{I}^{-}\right]$
Therefore, order of the reaction $=2$
Dimension of $\mathrm{k}=\frac{\text { Rate }}{\left[\mathrm{H}_{2} \mathrm{O}_{2}\right]\left[1^{-}\right]}$
$=\frac{\mathrm{mol} \mathrm{L}^{-1} \mathrm{~s}^{-1}}{\left(\mathrm{~mol} \mathrm{~L}^{-1}\right)\left(\mathrm{mol} \mathrm{L}^{-1}\right)}$
$=\mathrm{L} \mathrm{mol}^{-1} \mathrm{~s}^{-1}$
(iii) Given rate $=\mathrm{k}\left[\mathrm{CH}_{3} \mathrm{CHO}\right]^{\frac{3}{2}}$
Therefore, order of reaction $=\frac{3}{2}$
Dimension of $\mathrm{k}=\frac{\text { Rate }}{\left[\mathrm{H}_{2} \mathrm{O}_{2}\right]\left[1^{-}\right]}$
$=\frac{m o l L^{-1} s^{-1}}{\left(m o l L^{-1}\right)\left(m o l L^{-1}\right)}$
$=\mathrm{L} \mathrm{mol}^{-1} \mathrm{~s}^{-1}$
(iii) Given rate $=\mathrm{k}\left[\mathrm{CH}_{3} \mathrm{CHO}\right]^{\frac{3}{2}}$
Therefore, order of reaction $=\frac{3}{2}$
Dimension of $\mathrm{k}=\frac{\text { Rate }}{\left[\mathrm{CH}_{3} \mathrm{CHO}\right]^{\frac{3}{2}}}$
$=\frac{m o l L^{-1} s^{-1}}{\left(\operatorname{mol} L^{-1}\right)^{\frac{3}{2}}}$
$=\frac{\mathrm{mol} \mathrm{L}^{-1} \mathrm{~s}^{-1}}{\mathrm{~mol}^{\frac{3}{2}} \mathrm{~L}^{-\frac{3}{2}}}$
$=\mathrm{L}^{\frac{1}{2}} \mathrm{~mol}^{\frac{1}{2}} \mathrm{~s}^{-1}$
Question 2. For the reaction:
$2 \mathrm{~A}+\mathrm{B} \rightarrow \mathrm{A}_{2} \mathrm{~B}$ the rate $=\mathrm{k}[\mathrm{A}][\mathrm{B}]^{2}$ with $\mathrm{k}=2.0 \times 10^{-6} \mathrm{~mol}^{-2} \mathrm{~L}^{2} \mathrm{~s}^{-1} .$ Calculate
the initial rate of the reaction when $[\mathrm{A}]=0.1 \mathrm{~mol} \mathrm{~L}^{-1},[\mathrm{~B}]=0.2 \mathrm{~mol} \mathrm{~L}^{-1}$.
Calculate the initial rate of the reaction when $[\mathrm{A}]=0.1 \mathrm{~mol} \mathrm{~L}^{-1},[\mathrm{~B}]=$ $0.2 \mathrm{~mol} \mathrm{~L}^{-1} .$ Calculate the rate of reaction after $[\mathrm{A}]$ is reduced to $0.06 \mathrm{~mol} \mathrm{~L}^{-1}$.
Solution. Rate $=\mathrm{k}[\mathrm{A}][\mathrm{B}]^{2}$
rate constant $(\mathrm{k})=2.0 \times 10^{-6} \mathrm{~mol}^{-2} \mathrm{~L}^{2} \mathrm{~S}^{-1}$
$[\mathrm{A}]_{\text {initial }}=0.1 \mathrm{~mol} \mathrm{~L}^{-1}$
$[\mathrm{B}]_{\text {initial }}=0.2 \mathrm{~mol} \mathrm{~L}^{-1}$
We know
The initial rate of the reaction is $(\mathrm{r})=\mathrm{k}[\mathrm{A}][\mathrm{B}]^{2}$
$=\left(2.0 \times 10^{-6} \mathrm{~mol}^{-2} \mathrm{~L}^{2}\right)\left(0.1 \mathrm{~mol} \mathrm{~L}^{-1}\right)\left(0.2 \mathrm{~mol} \mathrm{~L}^{-1}\right)^{2}$
$=8.0 \times 10^{-9} \mathrm{~mol}^{-2} \mathrm{~L}^{2} \mathrm{~s}^{-1}$
When $[\mathrm{A}]$ is reduced from $0.1 \mathrm{~mol} \mathrm{~L}^{-1}$ to $0.06 \mathrm{~mol}^{-1}$,
The concentration of A reacted $=(0.1-0.06) \mathrm{mol} \mathrm{L}^{-1}=0.04 \mathrm{~mol} \mathrm{~L}^{-1}$
rate of reaction $=\frac{\mathrm{d}[\mathrm{A}]}{2 \mathrm{dt}}=\frac{\mathrm{d}[\mathrm{B}]}{\mathrm{dt}}$
Therefore, the concentration of B reacted $=\frac{\mathrm{d}[\mathrm{A}]}{2 \mathrm{dt}}=\frac{1}{2} \times 0.04 \mathrm{~mol} \mathrm{~L}^{-1}=$
$0.02 \mathrm{~mol} \mathrm{~L}^{-1}$
After $[\mathrm{A}]$ is reduced to $0.06 \mathrm{~mol} \mathrm{~L}^{-1}$, the rate of the reaction $=\mathrm{k}[\mathrm{A}][\mathrm{B}]^{2}=$ $\left(2.0 \times 10^{-6} \mathrm{~mol}^{-2} \mathrm{~L}^{2} \mathrm{~s}^{-1}\right)\left(0.06 \mathrm{~mol} \mathrm{~L}^{-1}\right)\left(0.18 \mathrm{~mol} \mathrm{~L}^{-1}\right)^{2}=3.89 \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}$
Question 3. The decomposition of $\mathrm{NH}_{3}$ on platinum surface is zero order reaction. What are the rates of production of $\mathrm{N}_{2}$ and $\mathrm{H}_{2}$ if $\mathrm{k}=2.5 \times 10^{-4} \mathrm{~mol}^{-1} \mathrm{Ls}^{-1}$ ?
Solution. Given:
The decomposition of $\mathrm{NH}_{3}$ on a platinum surface zero order reaction and showing below.
$2 \mathrm{NH}_{3}(\mathrm{~g}) \stackrel{\mathrm{Pt}}{\rightarrow} \mathrm{N}_{2}(\mathrm{~g})+3 \mathrm{H}_{2}(\mathrm{~g})$
We know, Rate of reaction $=-\frac{1}{2} \frac{\mathrm{d}\left[\mathrm{NH}_{3}\right]}{\mathrm{dt}}=\frac{\mathrm{d}\left[\mathrm{N}_{2}\right]}{\mathrm{dt}}=\frac{1}{3} \frac{\mathrm{d}\left[\mathrm{H}_{2}\right]}{\mathrm{dt}}$
However, $\mathrm{k}=2.5 \times 10^{-4} \mathrm{~mol}^{-1} \mathrm{Ls}^{-1}$
For zero order reaction, rate of reaction $=\mathrm{k}\left[\mathrm{NH}_{3}\right]^{0}$
rate of reaction $=\mathrm{k}$
Therefore,
Rate of reaction $=-\frac{1}{2} \frac{\mathrm{d}\left[\mathrm{NH}_{3}\right]}{\mathrm{dt}}=\frac{\mathrm{d}\left[\mathrm{N}_{2}\right]}{\mathrm{dt}}=\frac{1}{3} \frac{\mathrm{d}\left[\mathrm{H}_{2}\right]}{\mathrm{dt}}$
$=\mathrm{k}=2.5 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}$
Therefore, the rate of production of $\mathrm{N}_{2}$ is
$\frac{\mathrm{d}\left[\mathrm{N}_{2}\right]}{\mathrm{dt}}=2.5 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}$
And, the rate of production of $\mathrm{H}_{2}$ is
$\frac{\mathrm{d}\left[\mathrm{H}_{2}\right]}{\mathrm{dt}}=\frac{3 \mathrm{~d}\left[\mathrm{~N}_{2}\right]}{\mathrm{dt}}=3 \times 2.5 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}$
$=7.5 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}$
Question 4. The decomposition of dimethyl ether leads to the formation of $\mathrm{CH}_{4}, \mathrm{H}_{2}$ and $\mathrm{CO}$ and the reaction rate is given
Rate $=\mathrm{k}\left[\mathrm{CH}_{3} \mathrm{OCH}_{3}\right]^{\frac{3}{2}}$
If the pressure is measured in bar and time in minutes, then what are the units of rate and rate constants ?
Solution. Given:
Mentioned in question the pressure is in bar and time in minutes
We know, Rate $=[$ change in pressure $] /$ time $=[\mathrm{bar}] /$ time
units of rate $=$ bar $\min ^{-1}$
Rate $=\mathrm{k}\left[\mathrm{CH}_{3} \mathrm{OCH}_{3}\right]^{\frac{3}{2}}$
unit of rate constant $(\mathrm{k})=$ Rate $/\left[\mathrm{p}_{\mathrm{CH}_{3}} \mathrm{OCH}_{3}\right]^{\frac{3}{2}}=[\mathrm{bar}] /$ time $] /[\mathrm{bar}]^{\frac{3}{2}}=$
bar $\min ^{-1} /[\mathrm{bar}]^{\frac{3}{2}}$
$=\operatorname{bar}^{\frac{1}{2}} \min ^{-1}$
Concept insight: As $\mathrm{CH}_{3} \mathrm{OCH}_{3} \rightarrow \mathrm{CH}_{4}+\mathrm{H}_{2}+\mathrm{CO}$ thus the rate $=$ disappearance of diethyl ether
Question 5. Mention the factors that affect the rate of a chemical reaction.
Solution. These are the factors that affect the rate of a chemical 1. Concentration
2. Temperature
3. Presence or absence of a catalyst
4. Nature of reactants and products.
5. pH of the medium
6. The pressure inside a reaction container
7. Dielectric constant
8. Radiations / light
9. Electric / magnetic fields (charged / polar species)
Question 6. A reaction is second order with respect to a reactant. How is the rate of reaction affected if the concentration of the reactant is (i) doubled (ii) reduced to half?
Solution. $\mathrm{A} \rightarrow$ product
Let the concentration of the reactant $[\mathrm{A}]=\mathrm{x}$
It is mentioned in question reaction is second order with respect to a reactant so rate $\left(r_{1}\right)=k[A]^{2}=k[x]^{2}$
(i) If the concentration of reactant become double i.e. $[\mathrm{A}]=2 \mathrm{x}$
Then the rate of reaction would be $\left(\mathrm{r}_{2}\right)=\mathrm{k}[2 \mathrm{x}]^{2}=4 \mathrm{k}[\mathrm{x}]=4 \mathrm{r}_{1}$
Therefor the rate become four time increase as concentration become double
(ii) If the concentration of reactant become half i.e. $[\mathrm{A}]=\frac{\mathrm{x}}{2}$
Then the rate of reaction would be $\left(\mathrm{r}_{3}\right)=\mathrm{k}\left[\frac{\mathrm{x}}{2}\right]^{2}=\frac{\mathrm{k}[\mathrm{x}]}{4}=\frac{\mathrm{r}_{1}}{4}$
Therefor the rate become decrease by $\frac{1}{4}^{\text {th }}$ time as concentration become half
Question 7. What is the effect of temperature on the rate constant of a reaction? How can this temperature effect on rate constant be represented quantitatively?
Solution. Earlier the rate constant $(\mathrm{k})$ is nearly doubled with a rise in temperature by $10^{\circ}$ for a chemical reaction.
The temperature effect on the rate constant (k) can be represented quantitatively by the Arrhenius equation.
$\mathrm{k}=\mathrm{Ae}^{-\mathrm{E}_{\mathrm{a}} / \mathrm{RT}}$
Where $\mathrm{k}=$ the rate constant,
$\mathrm{A}=$ Arrhenius factor or the frequency factor,
$\mathrm{R}=$ gas constant
$\mathrm{T}=$ Temperature and
$\mathrm{E}_{\mathrm{a}}=$ The energy of activation for the reaction
Question 8. In a pseudo first hydrolysis of ester in water, the following results were obtained:
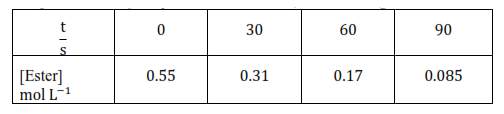
(i) Calculate the average rate of reaction between the time interval 30 to 60 seconds.
(ii) Calculated the pseudo first order rate constant for the hydrolysis of ester.
Solution: (i) At time 30 second to 60 -second Average rate of reaction between the time interval,
$=\frac{\mathrm{d}[\mathrm{Ester}]}{\mathrm{dt}}$
$=\frac{0.31-0.17}{60-30}$
$=4.67 \times 10^{-3} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}$
(ii) The formula used for pseudo-first-order reaction,
$\mathrm{k}=\frac{2.303}{\mathrm{t}} \log \frac{\left[\mathrm{R}_{0}\right]}{[\mathrm{R}]}$
(i) At $\mathrm{t}=30 \mathrm{~s},\left[\mathrm{R}_{0}\right]=0.55 ;[\mathrm{R}]=0.31$ so $\mathrm{k}_{1} \frac{2.303}{30} \log \frac{0.55}{0.31}=$
$1.911 \times 10^{-2} \mathrm{~s}^{-1}$
(ii) At $\mathrm{t}=60 \mathrm{~s},\left[\mathrm{R}_{0}\right]=0.55 ;[\mathrm{R}]=0.17$ so $\mathrm{k}_{2}=\frac{2.303}{60} \log \frac{0.55}{0.17}$
$=1.957 \times 10^{-2} \mathrm{~s}^{-1}$
(iii) $\quad$ At $\mathrm{t}=90 \mathrm{~s},\left[\mathrm{R}_{0}\right]=0.085 ;[\mathrm{R}]=0.17$ so $\mathrm{k}_{2}=\frac{2.303}{60} \log \frac{0.55}{0.085}$
$=2.705 \times 10^{-2} \mathrm{~s}^{-1}$
Then, the average rate constant $(\mathrm{k})=\frac{\mathrm{k}_{1}+\mathrm{k}_{2}+\mathrm{k}_{3}}{3}=$
$\frac{\left(1.911 \times 10^{-2}\right)+\left(1.957 \times 10^{-2}\right)+\left(2.075 \times 10^{-2}\right)}{3}$
$=1.98 \times 10^{-2} \mathrm{~s}^{-1}$
Question 9. A reaction is first order in A and second order in B.
(i) Write the differential rate equation
(ii) How is the rate affected on increasing the concentration of B three times?
(iii) How is the rate affected when the concentrations of both A and B are doubled?
Solution. (i) The differential rate equation will be
$\mathrm{r}_{1}=\frac{\mathrm{d}[\mathrm{R}]}{\mathrm{dt}}=\mathrm{k}[\mathrm{A}][\mathrm{B}]^{2}$
(ii) According to question the concentration of B is increased three times, then
$r_{2}=k[A][3 B]^{2}=9 \mathrm{k}[A][B]^{2}=9 r_{1}$
Therefore, the rate of reaction $\left(\mathrm{r}_{2}\right)$ will increase nine times than the initial rate.
(iii) According to the question, the concentrations of both A and B are doubled,
$\mathrm{r}_{1}=\mathrm{k}[\mathrm{A}][\mathrm{B}]^{2}$
$\mathrm{r}_{3}=\mathrm{k}[2 \mathrm{~A}][2 \mathrm{~B}]^{2}$
$r_{3}=8 \mathrm{k}[\mathrm{A}][\mathrm{B}]^{2}$
Therefore, the rate of reaction $\left(\mathrm{r}_{3}\right)$ will increase 8 times of initial rate $\left(\mathrm{r}_{1}\right) .$
Question 10. In a reaction between $\mathrm{A}$ and $\mathrm{B}$, the initial rate of reaction $\left(\mathrm{r}_{0}\right)$ was measured for different initial concentration of $\mathrm{A}$ and $\mathrm{B}$ as given below:
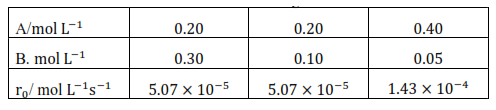
What is the order of the reaction with respect to A and B?
Solution: Assume; The order of the reaction with respect to $\mathrm{A}$ is $\mathrm{x}$ and with respect to $\mathrm{B}$ is $\mathrm{y}$.
Therefore, the rate of reaction $\left(\mathrm{r}_{0}\right)=\mathrm{k}[\mathrm{A}]^{\mathrm{x}}[\mathrm{B}]^{\mathrm{y}}$
$5.07 \times 10^{-5}=\mathrm{k}[0.20]^{\mathrm{x}}[0.30]^{\mathrm{y}} \quad$ (i)
$5.07 \times 10^{-5}=\mathrm{k}[0.20]^{\mathrm{x}}[0.10]^{\mathrm{y}}$( ii)
$1.43 \times 10^{-1}=\mathrm{k}[0.40]^{\mathrm{x}}[0.05]^{\mathrm{y}} \quad$ (iii)
Dividing equation (i) by (ii), we obtain
$\frac{5.07 \times 10^{-5}}{5.07 \times 10^{-5}}=\frac{\mathrm{k}[0.20]^{\mathrm{x}}[0.30]^{\mathrm{y}}}{\mathrm{k}[0.20]^{\mathrm{x}}[0.10]^{\mathrm{y}}}$
$\Rightarrow 1=\frac{[0.30]^{\mathrm{y}}}{[0.10]^{\mathrm{y}}}$
$\Rightarrow\left(\frac{0.30}{0.10}\right)^{0}=\left(\frac{0.30}{0.10}\right)^{\mathrm{y}}$
$\Rightarrow \mathrm{y}=0$
Dividing equation (iii) by (ii), we obtain
$\frac{1.43 \times 10^{-4}}{5.07 \times 10^{-5}}=\frac{\mathrm{k}[0.40]^{\mathrm{x}}[0.05]^{\mathrm{y}}}{\mathrm{k}[0.20]^{\mathrm{x}}[0.30]^{\mathrm{y}}}$
$\Rightarrow \frac{1.43 \times 10^{-1}}{5.07 \times 10^{-5}}=\frac{[0.40]^{\mathrm{x}}}{[0.20]^{\mathrm{z}}}\left[\begin{array}{c}\text { since } \mathrm{y}=0 \\ {[0.05]^{3}=[0.30]^{3}=1}\end{array}\right]$
$\Rightarrow 2.821=2^{\mathrm{x}}$
$\Rightarrow \log 2.821=\mathrm{xlog} 2$ (Taking log on both sides)
$\Rightarrow \mathrm{x}=\frac{\log 2.821}{\log 2}=1.496=1.5=\frac{3}{2}$
From the above calculation, we get the order of the reaction with respect to $\mathrm{A}=\frac{3}{2}$ and with respect to $\mathrm{B}=$ zero.
Question 11. The following results have been obtained during the kinetic studies of the reaction:
$2 \mathrm{~A}+\mathrm{B} \rightarrow \mathrm{C}+\mathrm{D}$
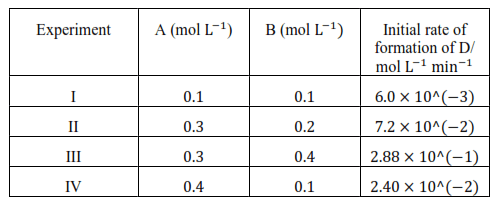
Determine the rate law and the rate constant for the reaction.
Solution: Assume the order of the reaction with respect to A is x and with respect to B is y.
Therefore, rate of the reaction is given by,
Rate $=\mathrm{k}[\mathrm{A}]^{\mathrm{x}}[\mathrm{B}]^{\mathrm{y}}$
According to the question,
$6.0 \times 10^{-3}=\mathrm{k}[0.1]^{\mathrm{x}}[0.1]^{\mathrm{y}} \quad \ldots$(i)
$7.2 \times 10^{-2}=\mathrm{k}[0.3]^{\mathrm{x}}[0.2]^{\mathrm{y}}$... (ii)
$2.88 \times 10^{-1}=\mathrm{k}[0.3]^{\mathrm{x}}[0.4]^{\mathrm{y}} \quad \ldots$(iii)
$2.40 \times 10^{-2}=\mathrm{k}[0.4]^{\mathrm{x}}[0.1]^{\mathrm{y}} \quad \ldots$ (iv)
Dividing equation (iv) by (i), we obtain
$\frac{2.40 \times 10^{-2}}{6.0 \times 10^{-3}}=\frac{\mathrm{k}[0.4]^{\mathrm{x}}[0.1]^{\mathrm{y}}}{\mathrm{k}[0.1]^{\mathrm{x}}[0.1]^{\mathrm{y}}}$
$\Rightarrow 4 \frac{[0.4]^{x}}{[0.1]^{x}}$
$\Rightarrow 4=\left(\frac{0.4}{0.1}\right)^{x}$
$\Rightarrow(4)^{1}=4^{x}$
$\Rightarrow x=1$
Dividing equation (iii) by (ii), we obtain
$\frac{2.88 \times 10^{-1}}{7.2 \times 10^{-2}}=\frac{\mathrm{k}[0.3]^{\mathrm{x}}[0.4]^{\mathrm{y}}}{\mathrm{k}[0.3]^{\mathrm{x}}[0.2]^{\mathrm{y}}}$
$\Rightarrow 4=\left(\frac{0.4}{0.2}\right)^{y}$
$\Rightarrow 4=2^{y}$
$\Rightarrow 2^{2}=2^{y}$
$\Rightarrow \mathrm{y}=2$
Therefore, the rate law is
Rate $=\mathrm{k}[\mathrm{A}][\mathrm{B}]^{2}$
From experiment (I), we get
$\mathrm{k}=\frac{6.0 \times 10^{-3} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~min}^{-1}}{\left(0.1 \mathrm{~mol} \mathrm{~L}^{-1}\right)\left(0.1 \mathrm{~mol} \mathrm{~L}^{-1}\right)^{2}}$
$=6.0 \mathrm{~L}^{2} \mathrm{~mol}^{-2} \mathrm{~min}^{-1}$
From experiment (II), we get
$\mathrm{k}=\frac{7.2 \times 10^{-2} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~min}^{-1}}{\left(0.3 \mathrm{~mol} \mathrm{~L}^{-1}\right)\left(0.2 \mathrm{~mol} \mathrm{~L}^{-1}\right)^{2}}$
$=6.0 \mathrm{~L}^{2} \mathrm{~mol}^{-2} \mathrm{~min}^{-1}$
From experiment (III), we get
$\mathrm{k}=\frac{2.88 \times 10^{-1} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~min}^{-1}}{\left(0.3 \mathrm{~mol} \mathrm{~L}^{-1}\right)\left(0.4 \mathrm{~mol} \mathrm{~L}^{-1}\right)^{2}}$
$=6.0 \mathrm{~L}^{2} \mathrm{~mol}^{-2} \mathrm{~min}^{-1}$
From experiment (IV) we get
$\mathrm{k}=\frac{2.40 \times 10^{-2} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~min}^{-1}}{\left(0.4 \mathrm{~mol} \mathrm{~L}^{-1}\right)\left(0.1 \mathrm{~mol} \mathrm{~L}^{-1}\right)^{2}}$
$=6.0 \mathrm{~L}^{2} \mathrm{~mol}^{-2} \mathrm{~min}^{-1}$
Therefore, the rate constant, $\mathrm{k}=6.0 \mathrm{~L}^{2} \mathrm{~mol}^{-2} \mathrm{~min}^{-1}$
Question 12. The reaction between $\mathrm{A}$ and $\mathrm{B}$ is first order with respect to $\mathrm{A}$ and zero order with respect to B. Fill in the blanks in the following table:
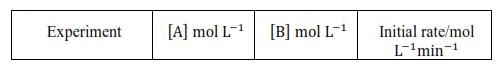
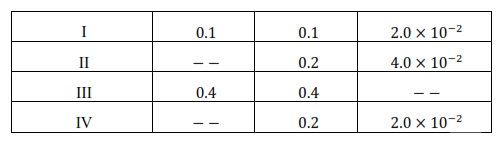
Solution. According to question the given reaction is of the first order with respect to A and of zero order with respect to B.
Therefore, the rate of the reaction is given by,
Rate $=\mathrm{k}[\mathrm{A}]^{1}[\mathrm{~B}]^{0}$
Rate $=\mathrm{k}[\mathrm{A}]$
From experiment II, we get
$2.0 \times 10^{-2} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~min}^{-1}=\mathrm{k}\left(0.1 \mathrm{~mol} \mathrm{~L}^{-1}\right)$
$\mathrm{k}=0.2 \mathrm{~min}^{-1}$
From experiment II, we get
$4.0 \times 10^{-2} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~min}^{-1}=0.2 \mathrm{~min}^{-1}[\mathrm{~A}]$
$[\mathrm{A}]=0.2 \mathrm{~mol} \mathrm{~L}^{-1}$
From experiment III, we get
Rate $=0.2 \mathrm{~min}^{-1} \times 0.4 \mathrm{~mol} \mathrm{~L}^{-1}$
$=0.08 \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~min}^{-1}$
From experiment IV, we get
$2.0 \times 10^{-2} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~min}^{-1}=0.2 \mathrm{~min}^{-1}[\mathrm{~A}]$
$[\mathrm{A}]=0.1 \mathrm{~mol} \mathrm{~L}^{-1}$

Concept Insight: First calculate the Rate constant and then putting each the value in different experiment we can calculate different values.
Question 13. Calculate the half-life of a first order reaction from their rate constants given below:
(i) $200 \mathrm{~s}^{-1}$
(ii) $2 \mathrm{~min}^{-1}$
(iii) 4 years $^{-1}$
Solution. According to question the unit of rate constant is time $^{-1}$
(i) $\quad$ Half life $\left(\mathrm{t}_{\frac{1}{2}}\right)=\frac{0.693}{\mathrm{k}}=\frac{0.693}{200 \mathrm{~s}^{-1}}$
$=3.47 \mathrm{~s}$ (approximately)
(ii) Half life, $\left(\mathrm{t}_{\frac{1}{2}}\right)=\frac{0.693}{\mathrm{k}}=\frac{0.693}{2 \mathrm{~min}^{-1}}$
$=0.35$ min (approximately)
(iii) Half life $\left(\mathrm{t}_{\frac{1}{2}}\right)=\frac{0.693}{\mathrm{k}}=\frac{0.693}{4 \text { years }^{-1}}$
$=0.173$ years (approximately)
Question 14. The half-life for radioactive decay of ${ }^{14} \mathrm{C}$ is 5730 years. An archaeological artifact containing wood had only $80 \%$ of the ${ }^{14} \mathrm{C}$ found in a living tree. Estimate the age of the sample.
Solution. Given: $\left[\mathrm{R}_{0}\right]=100$
$[\mathrm{R}]=80$
half-life for radioactive decay of ${ }^{14} \mathrm{C}=5730$ years
We know radioactive decay reaction is the first order
Here, $\mathrm{k}=\frac{0693}{\mathrm{t}_{\frac{1}{2}}}$
$=\frac{0.693}{5730}$ years $^{-1}$
It is known that,
$\mathrm{t}=\frac{2.303}{\mathrm{k}} \log \frac{[\mathrm{R}]_{0}}{[\mathrm{R}]}$
$=\frac{2.303}{\frac{0.693}{5730}} \log \frac{100}{80}$
$=1845$ years
Hence, the age of the sample is 1845 years.
Concept Insight: $80 \%$ of carbon found means that the initial value $\left[\mathrm{R}_{0}\right]$ of the $\mathrm{C}$ is 100 and that $[\mathrm{R}]$ is 80 .
Question 15. The experimental data for decomposition of $\mathrm{N}_{2} \mathrm{O}_{5}$
$\left[2 \mathrm{~N}_{2} \mathrm{O}_{5} \rightarrow 4 \mathrm{NO}_{2}+\mathrm{O}_{2}\right]$
In gas phase at $318 \mathrm{~K}$ are given below:

(i) Plot $\left[\mathrm{N}_{2} \mathrm{O}_{5}\right]$ against $\mathrm{t}$.
(ii) Find the half-life period for the reaction.
(iii) Draw a graph between $\log \left[\mathrm{N}_{2} \mathrm{O}_{5}\right]$ and $\mathrm{t}$.
(iv) What is the rate law?
(v) Calculate the rate constant.
(vi) Calculate the half-life period from k and compare it with (ii).
Solution: (i)
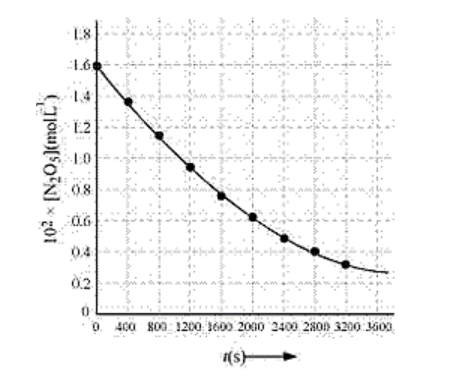
(ii) half life: time required for a substance to reduce to half of its initial concentration
$\mathrm{c}_{\mathrm{t}}=\frac{\mathrm{C}_{\mathrm{o}}}{2}=\frac{1.630 \times 10^{2}}{2} \mathrm{~mol} \mathrm{~L}^{-1}=81.5 \mathrm{~mol} \mathrm{~L}^{-1}$
is the half life. From thegraph, the half life is obtained as 1450 s.
(iii)
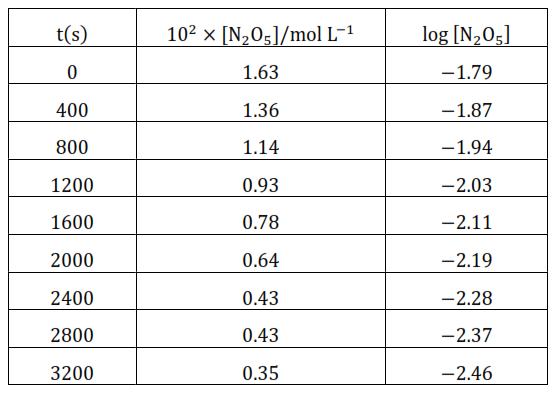
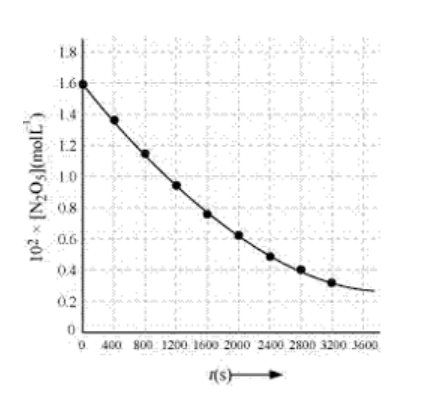
(iv) The given reaction is of the first order as the plot, $\log \left[\mathrm{N}_{2} \mathrm{O}_{5}\right] \mathrm{v} / \mathrm{s} \mathrm{t}$, is straight line. Therefore, the rate law of the reaction is
Rate $=\mathrm{k}\left[\mathrm{N}_{2} \mathrm{O}_{5}\right]$
(v) From the plot, $\log \left[\mathrm{N}_{2} \mathrm{O}_{5}\right] \mathrm{v} / \mathrm{s} \mathrm{t}$, we get
Slope $=\frac{-2.46-(-1.79)}{3200-0}=\frac{-0.67}{3200}$
Again, slope of the line of the plot $\log \left[\mathrm{N}_{2} \mathrm{O}_{5}\right] \mathrm{v} / \mathrm{s}$ time $(\mathrm{t})$ is given by
$\Rightarrow-\frac{\mathrm{k}}{2.303}=-\frac{0.67}{3200}$
$\Rightarrow \mathrm{k}=4.82 \times 10^{-4} \mathrm{~s}^{-1}$
(vi) Half-lift is given by,
$\mathrm{t}_{\frac{1}{2}}=\frac{0.639}{\mathrm{k}}$
$=\frac{0.693}{4.82 \times 10^{-4}} \mathrm{~s}$
$=\frac{0.693}{4.82 \times 10^{-4}} \mathrm{~s}$
$=1.438 \times 10^{3} \mathrm{~s}$
$=1438 \mathrm{~s}$
This value, $1438 \mathrm{~s}$, is very close to the value that was obtained from the graph.
Question 16. The rate constant for a first order reaction is $60 \mathrm{~s}^{-1}$. How much time will it take to reduce the initial concentration of the reactant to its $\frac{1}{16}$ value?
Solution. Give : rate constant $(\mathrm{k})$ for a first order reaction $=60 \mathrm{~s}^{-1}$
Let assume: initial concentration $[\mathrm{R}]_{0}=\mathrm{x}$
Final concentration $[R]=\frac{x}{16^{\prime}}$ reduce the initial concentration of the reactant to its
$\frac{1}{16}$ th
Formula ,
$\mathrm{t}=\frac{2.303}{\mathrm{k}} \log \frac{[\mathrm{R}]_{0}}{[\mathrm{R}]}$ for first order reaction
$=\frac{2.303}{\mathrm{k}} \log \frac{\mathrm{x}}{\frac{\mathrm{X}}{16}}=\frac{2.303}{\mathrm{k}} \log \frac{1}{\frac{1}{16}}$
$=\frac{2.303}{60 \mathrm{~s}^{-1}} \log 16$
$=4.6 \times 10^{-2} \mathrm{~S}$ (approximately)
Hence, the required time is $4.6 \times 10^{-2} \mathrm{~s}$.
Concept Insight: If the initial concentration is one than the final concentration is $\frac{1}{16} .$
Question 17. During nuclear explosion, one of the products is ${ }^{90} \mathrm{Sr}$ with half-life of $28.1$ years. If $1 \mu \mathrm{g}$ of ${ }^{90} \mathrm{Sr}$ was absorbed in the bones of a newly born baby instead of calcium, how much of it will remain after 10 years and 60 years and 60 years if it is not lost metabolically.
Solution: Given: half-life $=28.1$ years
${ }^{90} \mathrm{Sr}$ was absorbed in the bones $=1 \mu \mathrm{g}$
Here, $\mathrm{k}=\frac{0.693}{\mathrm{t}_{\frac{1}{2}}}=\frac{0.693}{28.1} \mathrm{y}^{-1}$
Formula,
$\mathrm{t}=\frac{2.303}{\mathrm{k}} \log \frac{[\mathrm{R}]_{0}}{[\mathrm{R}]}$ for first order reaction
$\Rightarrow{ }^{90} \mathrm{Sr}$ will remain after 10 years
$t=\frac{2.303}{\frac{0.693}{28.1}} \log \frac{1}{[\mathrm{R}]}$
$\Rightarrow 10=\frac{2.303}{\frac{0.693}{28.1}} \log \frac{1}{[\mathrm{R}]}$
$\Rightarrow 10=\frac{2.303}{\frac{0.693}{28.1}}(-\log [\mathrm{R}])$
$\Rightarrow \log [\mathrm{R}]=-\frac{10 \times 0.693}{2.303 \times 28.1}$
$\Rightarrow[\mathrm{R}]=\operatorname{anti} \log (-0.1071)$
$\Rightarrow[\mathrm{R}]=\operatorname{antilog}(-0.1071)$
$=\operatorname{antilog}(\overline{1} .8929)$
$=0.7814 \mu \mathrm{g}$
Therefore, $0.7814 \mu \mathrm{g}$ of ${ }^{90} \mathrm{Sr}$ will remain after 10 years.
$\Rightarrow{ }^{90} \mathrm{Sr}$ will remain after 60 years
Formula used Again, $\mathrm{t}=\frac{2.303}{\mathrm{k}} \log \frac{[\mathrm{R}]_{0}}{[\mathrm{R}]}$
$\Rightarrow 60=\frac{2.303}{\frac{0.693}{28.1}} \log \frac{1}{[\mathrm{R}]}$
$\Rightarrow \log [R]=-\frac{60 \times 0.693}{2.303 \times 28.1}$
$\Rightarrow[\mathrm{R}]=\operatorname{antilog}(-0.6425)$
$=\operatorname{antilog}(\overline{1} .3575)$
$=0.2278 \mu \mathrm{g}$
Therefore, $0.2278 \mu \mathrm{g}$ of ${ }^{90} \mathrm{Sr}$ will remain after 60 years.
Question 18. For a first order reaction, show that time required for $99 \%$ completion is twice the time required for the completion of $90 \%$ of reaction.
Solution: Given: order reaction $=$ first
Reaction complete $99 \%$ then
We know $[\mathrm{R}]_{0}=100 ;[\mathrm{R}]=100-99$
We know that, $\mathrm{t}=\frac{2.303}{\mathrm{k}} \log \frac{[\mathrm{R}]_{0}}{[\mathrm{R}]}$
The time required for $99 \%$ completion $\left(\mathrm{t}_{1}\right)$
$=\frac{2.303}{\mathrm{k}} \log \frac{100}{100-99}$
$=\frac{2.303}{\mathrm{k}} \log 100$
$=2 \times \frac{2.303}{\mathrm{k}}$ equation
Reaction Complete $90 \%$ then
We know $[\mathrm{R}]_{0}=100 ;[\mathrm{R}]=100-90$
The time required for $90 \%$ completion is $\left(t_{2}\right)=\frac{2.303}{k} \log \frac{100}{100-90}$
$=\frac{2.303}{\mathrm{k}} \log 10$
$=\frac{2.303}{\mathrm{k}}$ equation 2
Equation 1 devide by equation 2 we get $t_{1}=2 t_{2}$
Hence, first order reaction the time required for $99 \%$ completion of a is twice the time required for the completion of $90 \%$.
Concept Insight: Let initial concentration be 'a' than the final concentration is ' $\mathrm{a}-\mathrm{x}^{\prime}$ That is $[\mathrm{R}]_{0}=100$ than $[\mathrm{R}]=100-90$
Question 19. A first order reaction takes 40 min for $30 \%$ decomposition. Calculate $t_{\frac{1}{2}}$.
Solution. Given: order of reaction $=$ first
$[\mathrm{R}]_{0}=100 ;[\mathrm{R}]=100-30=70$
Formula: $\mathrm{t}=\frac{2.303}{\mathrm{k}} \log \frac{[\mathrm{R}]_{0}}{[\mathrm{R}]}$
$\mathrm{k}=\frac{2.303}{40 \mathrm{~min}} \log \frac{100}{70}$
$=\frac{2.303}{40 \mathrm{~min}} \log \frac{10}{7}$
$=8.9168 \times 10^{-3} \mathrm{~min}^{-1}$
Formula for $\mathrm{t}_{1}$ of the decomposition reaction for $=\frac{0.693}{\mathrm{k}}$
$=\frac{0.693}{8.916 \times 10^{-3}} \mathrm{~min}=77.71 \mathrm{~min}$
Question 20. For the decomposition of azoisopropane to hexane and nitrogen at $543 \mathrm{~K}$, the following data are obtained.
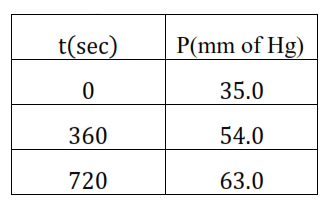
Calculate the rate constant.
Solution. The decomposition of azoisopropane to hexane and nitrogen at $543 \mathrm{~K}$ is first order reaction which is represented by the following equation.

After time, $t$, total pressure, $P_{t}=\left(P_{0}-p\right)+p+p$
$\Rightarrow P_{t}=P_{0}+p$
$\Rightarrow \mathrm{p}=\mathrm{P}_{\mathrm{t}}-\mathrm{P}_{0}$
Therefore, $P_{0}-p=P_{0}-\left(P_{t}-P_{0}\right)$
$=2 \mathrm{P}_{0}-\mathrm{P}_{\mathrm{t}}$
Formula: for a first order reaction $\mathrm{k}=\frac{2.303}{\mathrm{t}} \log \frac{[\mathrm{R}]_{0}}{[\mathrm{R}]}$
Where $[\mathrm{R}]_{0}=\mathrm{P}_{0} ; \quad[\mathrm{R}]=\mathrm{P}_{0}-\mathrm{p}$
$\mathrm{k}=\frac{2.303}{\mathrm{t}} \log \frac{\mathrm{P}_{0}}{\mathrm{P}_{0}-\mathrm{p}}$
$=\frac{2.303}{\mathrm{t}} \log \frac{\mathrm{P}_{0}}{2 \mathrm{P}_{0}-\mathrm{P}_{\mathrm{t}}}$
At $t=360 \mathrm{~s}, \mathrm{k}=\frac{2.303}{360 \mathrm{~s}} \log \frac{(35.0)}{(2 \times 35.0-54.0)}$
$=2.175 \times 10^{-3} \mathrm{~s}^{-1}$
At $t=720 \mathrm{~s}, \mathrm{k}=\frac{2.303}{7020 \mathrm{~s}} \log \frac{(35.0)}{(2 \times 35.0-63.0)}$
$=2.235 \times 10^{-3} \mathrm{~s}^{-1}$
Hence, the average value of rate constant is
$\mathrm{k}=\frac{\left(2.175 \times 10^{-3}\right)+\left(2.235 \times 10^{-3}\right)}{2} \mathrm{~s}^{-1}$
$=2.21 \times 10^{-3} \mathrm{~S}^{-1}$
Note: There is a slight variation in this answer and the one given in the NCERT
text book.
Question 21. The following data were obtained during the first order thermal decomposition of $\mathrm{SO}_{2} \mathrm{Cl}_{2}$ at a constant volume.
$\mathrm{SO}_{2} \mathrm{Cl}_{2}(\mathrm{~g}) \rightarrow \mathrm{SO}_{2}(\mathrm{~g})+\mathrm{Cl}_{2}(\mathrm{~g})$

Calculate the rate of the reaction when total pressure is $0.65 \mathrm{~atm}$.
Solution. The thermal decomposition of $\mathrm{SO}_{2} \mathrm{Cl}_{2}$ at a constant volume is represented by the following equation.

After time, $\mathrm{t}$, total pressure, $\mathrm{P}_{\mathrm{t}}=\left(\mathrm{P}_{0}-\mathrm{p}\right)+\mathrm{p}+\mathrm{p}$
$\Rightarrow \mathrm{P}_{\mathrm{t}}=\mathrm{P}_{0}+\mathrm{p}$
$\Rightarrow \mathrm{p}=\mathrm{P}_{\mathrm{t}}-\mathrm{P}_{0}$
Therefore, $\mathrm{P}_{0}-\mathrm{p}=\mathrm{P}_{0}-\left(\mathrm{P}_{\mathrm{t}}-\mathrm{P}_{0}\right)$
$=2 \mathrm{P}_{0}-\mathrm{P}_{\mathrm{t}}$
Formula: for a first order reaction rate constant $(\mathrm{k})=\frac{2.303}{\mathrm{t}} \log \frac{[\mathrm{R}]_{0}}{[\mathrm{R}]}$
Where $[R]_{0}=P_{0} ;[R]=2 P_{0}-p$
$\mathrm{k}=\frac{2.303}{\mathrm{t}} \log \frac{\mathrm{P}_{0}}{2 \mathrm{P}_{0}-\mathrm{p}}$
$=\frac{2.303}{t} \log \frac{P_{0}}{2 P_{0}-P_{t}}$
When $\mathrm{t}=100 \mathrm{~s}, \mathrm{k}=\frac{2.303}{100} \log \frac{(0.5)}{(2 \times 0.5-0.6)}$
$=2.231 \times 10^{-3} \mathrm{~s}^{-1}$
When $\mathrm{P}_{\mathrm{t}}=0.65 \mathrm{~atm}$
$\mathrm{P}_{0}+\mathrm{p}=0.65$
$\mathrm{P}_{0}=0.5$
$\Rightarrow \mathrm{p}=0.65-\mathrm{P}_{0}$
$p=0.65-0.5$
$p=0.15 \mathrm{~atm}$
Therefore, when the total pressure is $0.65$ atm, pressure of $\mathrm{SOCl}_{2}\left(\mathrm{P}_{\mathrm{SOCl}_{2}}\right)=$
$\mathrm{P}_{0}-\mathrm{p}=0.5-0.15$
$=0.35 \mathrm{~atm}$
when total pressure is $0.65 \mathrm{~atm}$, the rate of equation $(\mathrm{r})=\mathrm{k}\left(\mathrm{P}_{\mathrm{SOCl}_{2}}\right)$
$=\left(2.23 \times 10^{-3} \mathrm{~s}^{-1}\right)(0.35 \mathrm{~atm})$
$=7.8 \times 10^{-4} \mathrm{~atm} \mathrm{~s}^{-1}$
Question 22. The rate constant for the decomposition of $\mathrm{N}_{2} \mathrm{O}_{5}$ at various temperatures is given below:

Draw a graph between in $\mathrm{k}$ and $1 / \mathrm{T}$ and calculate the values of $\mathrm{A}$ and $\mathrm{E}_{\mathrm{a}}$.
Predict the rate constant at $30^{\circ}$ and $50^{\circ} \mathrm{C}$.
Solution. From the given data, we obtain



Slope of the line,
$\frac{y_{2}-y_{1}}{x_{2}-x_{1}}=12.301 \mathrm{~K}$
According to Arrhenius equation,
Slope $=-\frac{\mathrm{E}_{\mathrm{a}}}{\mathrm{R}}$
$\Rightarrow \mathrm{E}_{\mathrm{a}}=-$ Slope $\times \mathrm{R}$
$=-(-12.301 \mathrm{~K}) \times\left(8.314 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}\right)$
$=102.27 \mathrm{~kJ} \mathrm{~mol}^{-1}$
Again,
In $\mathrm{k}=\operatorname{In} \mathrm{A}-\frac{\mathrm{E}_{\mathrm{a}}}{\mathrm{RT}}$
$\operatorname{In} A=\operatorname{In} k+\frac{E_{a}}{R T}$
When $\mathrm{T}=273 \mathrm{~K}$
In $\mathrm{k}=-7.147$
Then, In $\mathrm{A}=-7.147+\frac{102.27 \times 10^{3}}{8.314 \times 273}$
$=37.911$
Therefore, $A=2.91 \times 10^{6}$
When $\mathrm{T}=30+273 \mathrm{~K}=303 \mathrm{~K}$
$\frac{1}{\mathrm{~T}}=0.0033 \mathrm{~K}=3.3 \times 10^{-3} \mathrm{~K}$
Then, at $\frac{1}{\mathrm{~T}}=3.3 \times 10^{-3} \mathrm{~K}$,
In $\mathrm{k}=-2.8$
Therefore, $\mathrm{k}=6.08 \times 10^{-2} \mathrm{~s}^{-1}$
Again, when $\mathrm{T}=50+273 \mathrm{~K}=323 \mathrm{~K}$
$\frac{1}{\mathrm{~T}}=0.0031 \mathrm{~K}=3.1 \times 10^{-3} \mathrm{~K}$
\text { Then, at } \frac{1}{\mathrm{~T}}=3.1 \times 10^{-3} \mathrm{~K},
In $\mathrm{k}=-0.5$
Therefore, $\mathrm{k}=0.607 \mathrm{~s}^{-1}$
Question 23. The rate constant for the decomposition of hydrocarbons is $2.418 \times 10^{-5} \mathrm{~s}^{-1}$ at $546 \mathrm{~K}$. If the energy of activation is $179.9 \frac{\mathrm{kJ}}{\mathrm{mol}}$, what will be the value of preexponential factor.
Solution. Given: rate constant $(\mathrm{k})=2.418 \times 10^{-5} \mathrm{~s}^{-1}$ it is first order reaction
$\mathrm{T}=546 \mathrm{~K}$ $\mathrm{E}_{\mathrm{a}}=179.9 \mathrm{~kJ} \mathrm{~mol}^{-1}=179.9 \times 10^{3} \mathrm{~J} \mathrm{~mol}^{-1}$
According to the Arrhenius equation,
$\mathrm{k}=\mathrm{Ae}^{\mathrm{E}_{\mathrm{a}} / \mathrm{RT}}$
$\Rightarrow \operatorname{In} \mathrm{k}=\operatorname{In} \mathrm{A}-\frac{\mathrm{E}_{\mathrm{a}}}{\mathrm{RT}}$
$\Rightarrow \log \mathrm{k}=\log \mathrm{A}-\frac{\mathrm{E}_{\mathrm{a}}}{2.303 \mathrm{RT}}$
$\Rightarrow \log \mathrm{A}=\log \mathrm{k}+\frac{\mathrm{E}_{\mathrm{a}}}{2.303 \mathrm{RT}}$
$=\log \left(2.418 \times 10^{-5} \mathrm{~s}^{-1}\right)$
$+\frac{179.9 \times 10^{3} \mathrm{~J} \mathrm{~mol}^{-1}}{2.303 \times 8.314 \mathrm{JK}^{-1} \mathrm{~mol}^{-1} \times 546 \mathrm{~K}}$
$=(0.3835-5)+17.2082$
$=12.5917$
Therefore, $\mathrm{A}=\operatorname{antilog}(12.5917)$
$3.9 \times 10^{12} \mathrm{~s}^{-1}$
Concept Insight: 'A' is called Arrhenius factor, frequency factor or preexponential factor.
Question 24. Consider a certain reaction $\mathrm{A} \rightarrow$ Products with $\mathrm{k}=2.0 \times 10^{-2} \mathrm{~s}^{-1} .$ Calculate the concentration of A remaining after $100 \mathrm{~s}$ if the initial concentration of $\mathrm{A}$ is $1.0 \mathrm{~mol} \mathrm{~L}^{-1}$.
Solution. Given: $\mathrm{k}=2.0 \times 10^{-2} \mathrm{~s}^{-1}$
$\mathrm{T}=100 \mathrm{~s}$
$[\mathrm{A}]_{0}=1.0 \mathrm{~mol} \mathrm{~L}^{-1}$
Since the unit of $\mathrm{k}$ is $\mathrm{s}^{-1}$, the given reaction is a first order reaction.
Formula: $\mathrm{k}=\frac{2.303}{\mathrm{t}} \log \frac{[\mathrm{R}]_{0}}{[\mathrm{R}]}$
$\Rightarrow 2.0 \times 10^{2} \mathrm{~S}^{-1}=\frac{2.303}{100 \mathrm{~s}} \log \frac{1.0}{[\mathrm{R}]}$
$\Rightarrow 2.0 \times 10^{-2} \mathrm{~S}^{-1}=\frac{2.303}{100 \mathrm{~s}}(\log [\mathrm{R}])$
$=-\log [\mathrm{R}]=\frac{2.0 \times 10^{-2} \times 100}{2.303}$
$\Rightarrow[\mathrm{R}]=\operatorname{antilog}\left(-\frac{2.0 \times 10^{-2} \times 100}{2.303}\right)$
$=0.135 \mathrm{~mol} \mathrm{~L}^{-1}$ (approximately)
Hence the remaining concentration of $\mathrm{A}$ is $0.135 \mathrm{~mol} \mathrm{~L}^{-1}$.
Concept insight: since the value of $\mathrm{k}$ is $\mathrm{s}^{-1}$. Hence it is a first order reaction. So looking at the units of the rate constant we can find out the order of reaction.
Question 25. Sucrose decomposes in acid solution into glucose and fructose according to the first order rate law, with $\mathrm{t}_{\frac{1}{2}}=3.00$ hours. What fraction of sample of sucrose?
remains after 8 hours?
Solution: Given:
Order of reaction: first
Half life $\mathrm{t}_{\frac{1}{2}}=3.00$ hours
Formula: For a first order reaction rate constant $(\mathrm{k})=\frac{2.303}{\mathrm{t}} \log \frac{[\mathrm{R}]_{0}}{[\mathrm{R}]}$
We know rate constant $(\mathrm{k})=\frac{0.693}{\mathrm{t}_{\frac{1}{2}}}=\frac{0.693}{3} \mathrm{~h}^{-1}=0.231 \mathrm{~h}^{-1}$
Then, $0.231 \mathrm{~h}^{-1}=\frac{2.303}{8 \mathrm{~h}} \log \frac{[\mathrm{R}]_{0}}{[\mathrm{R}]}$
$\Rightarrow \log \frac{[\mathrm{R}]_{0}}{[\mathrm{R}]}=\frac{0.231 \mathrm{~h}^{-1} \times 8 \mathrm{~h}}{2.303}$
$\Rightarrow \frac{[\mathrm{R}]_{0}}{[\mathrm{R}]}=$ antilog $(0.8024)$
$\Rightarrow \frac{[\mathrm{R}]_{0}}{[\mathrm{R}]}=6.3445$
$\Rightarrow \frac{[\mathrm{R}]_{0}}{[\mathrm{R}]}=0.158$
Hence, the fraction of sample of sucrose that remains after 8 hours is $0.158$.
Question 26. The decomposition of hydrocarbon follows the equation $\mathrm{k}=(4.5 \times$ $\left.10^{11} \mathrm{~s}^{-1}\right) \mathrm{e}^{-28000 \mathrm{~K} / \mathrm{T}}$
Calculate $E_{a}$
Solution. The given equation is
$\mathrm{k}=\left(4.5 \times 10^{11} \mathrm{~s}^{-1}\right) \mathrm{e}^{-28000 \mathrm{~K} / \mathrm{T}} \quad$ equation (i)
$\mathrm{k}=\mathrm{Ae}^{-\frac{\mathrm{E}_{\mathrm{a}}}{\mathrm{RT}}}$ equation (ii)
As compare the equation (i) and (ii), we get
$\frac{\mathrm{E}_{\mathrm{a}}}{\mathrm{RT}}=\frac{28000 \mathrm{~K}}{\mathrm{~T}}$
$\Rightarrow \mathrm{E}_{\mathrm{a}}=\mathrm{R} \times 28000 \mathrm{~K}$
$=8.314 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1} \times 28000 \mathrm{~K}$
$=232792 \mathrm{Jmol}^{-1}$
$=232.792 \mathrm{~kJ} \mathrm{~mol}^{-1}$
or $E_{a}=R \times 28000 K$
$=2 \mathrm{cal} \mathrm{K}^{-1} \mathrm{~mol}^{-1} \times 28000 \mathrm{~K}$
$56000 \mathrm{Jmol}^{-1}$
$=56 \mathrm{~kJ} \mathrm{~mol}^{-1}$
Question 27. The rate constant for the first order decomposition of $\mathrm{H}_{2} \mathrm{O}_{2}$ is given by the following equation:
Solution. Given: $\log \mathrm{k}=14.34-1.25 \times 10^{4} \mathrm{~K} / \mathrm{T}$
Half life $=256$ minute
Arrhenius equation is given by,
$\mathrm{k}=\mathrm{Ae}^{-\mathrm{E}_{\mathrm{a}} / \mathrm{RT}}$
$\Rightarrow \operatorname{In} \mathrm{k}=\operatorname{In} \mathrm{A}-\frac{\mathrm{E}_{\mathrm{a}}}{\mathrm{RT}}$
$\Rightarrow \log \mathrm{k}=\log \mathrm{A}-\frac{\mathrm{E}_{\mathrm{a}}}{2.303 \mathrm{RT}} \quad$ equation
given in question: $\log \mathrm{k}=14.34-1.25 \times 10^{4} \mathrm{~K} / \mathrm{T}$ equation (ii)
From equation (i) and (ii), we obtain
$\frac{\mathrm{E}_{\mathrm{a}}}{2.303 \mathrm{RT}}=\frac{1.25 \times 10^{4} \mathrm{~K}}{\mathrm{~T}}$
$\Rightarrow \mathrm{E}_{\mathrm{a}}=1.25 \times 10^{4} \mathrm{~K} \times 2.303 \times \mathrm{R}$
$=1.25 \times 10^{4} \mathrm{~K} \times 2.303 \times 8.314 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}$
$=239.34 \mathrm{~kJ} \mathrm{~mol}^{-1}$
Also, when $\mathrm{t}_{\frac{1}{2}}=256$ minutes,
We know rate constant $(\mathrm{k})=\frac{0.693}{\mathrm{t}_{1 / 2}}$
$=\frac{0.693}{256}=2.707 \times 10^{-3} \mathrm{~min}^{-1}$
$=\frac{2.707 \times 10^{-3}}{60} \mathrm{sec}^{-1}$
$=4.51 \times 10^{-5} \mathrm{~s}^{-1}$
It is also given that, $\log \mathrm{k}=14.34-1.25 \times 10^{4} \mathrm{~K} / \mathrm{T}$
$\Rightarrow \log \left(4.51 \times 10^{-5}\right)=14.34-\frac{1.25 \times 10^{4} \mathrm{~K}}{\mathrm{~T}}$
$\Rightarrow \log (0.654-05)=14.34-\frac{1.25 \times 10^{4} \mathrm{~K}}{\mathrm{~T}}$
$\Rightarrow \frac{1.25 \times 10^{4} \mathrm{~K}}{\mathrm{~T}}=18.686$
$\Rightarrow \mathrm{T}=\frac{1.25 \times 10^{4} \mathrm{~K}}{18.686}$
$=668.95 \mathrm{~K}$
$=669 \mathrm{~K}$ (approximately)
Question 28. The decomposition of $\mathrm{A}$ into product has value of $\mathrm{k}$ as $4.5 \times 10^{3} \mathrm{~s}^{-1}$ at $10^{\circ} \mathrm{C}$ and energy of activation of $60 \mathrm{~kJ} \mathrm{~mol}^{-1} .$ At what temperature would $\mathrm{k}$ be $1.5 \backslash$ time $10^{4} \mathrm{~s}^{-1}$ ?
Solution. Given: $\mathrm{k}_{1}=4.5 \times 10^{3} \mathrm{~s}^{-1}$
$\mathrm{T}_{1}=273+10=283 \mathrm{~K}$
$\mathrm{k}_{2}=1.5 \times 10^{4} \mathrm{~s}^{-1}$
$\mathrm{E}_{\mathrm{a}}=60 \mathrm{~kJ} \mathrm{~mol}^{-1}=6.0 \times 10^{4} \mathrm{~J} \mathrm{~mol}^{-1}$
Formula: Arrhenius equation, $\log \frac{\mathrm{k}_{2}}{\mathrm{k}_{1}}=\frac{\mathrm{E}_{\mathrm{a}}}{2.303 \mathrm{R}}\left(\frac{\mathrm{T}_{2}-\mathrm{T}_{1}}{\mathrm{~T}_{1} \mathrm{~T}_{2}}\right)$
Put the given values in formula we get
$\log \frac{1.5 \times 10^{4}}{4.5 \times 10^{3}}=\frac{6.0 \times 10^{4} \mathrm{~J} \mathrm{~mol}^{-1}}{2.303 \times 8.314 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}}\left(\frac{\mathrm{T}_{2}-283}{283 \mathrm{~T}_{2}}\right)$
$\Rightarrow 0.5229=3133.627\left(\frac{\mathrm{T}_{2}-283}{283 \mathrm{~T}_{2}}\right)$
$\Rightarrow \frac{0.5229 \times 283 \mathrm{~T}_{2}}{3133.627}=\mathrm{T}_{2}-283$
$\Rightarrow 0.0472 \mathrm{~T}_{2}=\mathrm{T}_{2}-283$
$\Rightarrow 0.9528 \mathrm{~T}_{2}=283$
$\Rightarrow \mathrm{T}_{2}=297.019 \mathrm{~K}$ (approximately)
$=297 \mathrm{~K}$
$297 \mathrm{~K}-273 \mathrm{k}=24^{\circ} \mathrm{C}$
Hence, $\mathrm{k}$ would be $1.5 \times 10^{4} \mathrm{~s}^{-1}$ at $24^{\circ} \mathrm{C}$.
Question 29. The time required for $10 \%$ completion of a first order reaction at $298 \mathrm{~K}$ is equal to that required for its $25 \%$ completion at $308 \mathrm{~K}$. if the value of $\mathrm{A}$ is $4 \times 10^{10} \mathrm{~s}^{-1}$. Calculate $\mathrm{k}$ at $318 \mathrm{~K}$ and $\mathrm{E}_{\mathrm{a}}$.
Solution. Given: order of reaction $=$ first
Temperature $(\mathrm{t})=298 \mathrm{k} ;$ reaction completion $=10 \%$
Temperature $\left(\mathrm{t}^{\prime}\right)=308 \mathrm{k} ;$ reaction completion $=25 \%$
Temperature $\left(\mathrm{t}_{3}\right)=318 \mathrm{k}$
$A=4 \times 10^{10} \mathrm{~S}^{-1}$
For a first order reaction,
$\mathrm{t}=\frac{2.303}{\mathrm{k}} \log \frac{\left[\mathrm{R}_{0}\right]}{[\mathrm{R}]}$
The time required for $10 \%$ completion of a first order reaction at $298 \mathrm{~K}$ :
$\left[\mathrm{R}_{0}\right]=100 ;[\mathrm{R}]=100-10=90$
$\mathrm{t}=\frac{2.303}{\mathrm{k}} \log \frac{100}{90}$
$=\frac{0.1054}{\mathrm{k}}$
The time required for $25 \%$ completion of a first order reaction at $398 \mathrm{~K}$ :
$\left[\mathrm{R}_{0}\right]=100 ;[\mathrm{R}]=100-25=75$
$\mathrm{t}^{\prime}=\frac{2.303}{\mathrm{k}^{\prime}} \log \frac{100}{75}$
$=\frac{2.2877}{\mathrm{k}^{\prime}}$
According to the question
$\mathrm{t}=\mathrm{t}^{\prime}$
$\Rightarrow \frac{0.1054}{\mathrm{k}}=\frac{0.2877}{\mathrm{k}^{\prime}}$
$\Rightarrow \frac{\mathrm{k}^{\prime}}{\mathrm{k}}=2.7296$
From Arrhenius equation, we obtain
$\log \frac{\mathrm{k}^{\prime}}{\mathrm{k}}=\frac{\mathrm{E}_{\mathrm{a}}}{2.303 \mathrm{R}}\left(\frac{\mathrm{T}^{\prime}-\mathrm{T}}{\mathrm{TT}^{\prime}}\right)$
$\log (2.7296)=\frac{\mathrm{E}_{\mathrm{a}}}{2.303 \times 8.314}\left(\frac{308-298}{298 \times 308}\right)$
$E_{a}=\frac{2.303 \times 8.314 \times 298 \times 308 \times \log (2.7296)}{308-298}$
$=76640.096 \mathrm{~J} \mathrm{~mol}^{-1}$
$=76.64 \mathrm{~kJ} \mathrm{~mol}^{-1}$
To calculate $\mathrm{k}$ at $318 \mathrm{~K}$,
It is given that, $\mathrm{A}=4 \times 10^{10} \mathrm{~s}^{-1}, \mathrm{~T}_{3}=318 \mathrm{~K}$
Again, from Arrhenius equation, we obtain
$\log \mathrm{k}=\log \mathrm{A}-\frac{\mathrm{E}_{\mathrm{a}}}{2.303 \mathrm{RT}_{3}}$
$=\log \left(4 \times 10^{10}\right)-\frac{76.64 \times 10^{3}}{2.303 \times 8.314 \times 318}$
$=(0.6021+10)-12.5876$
$=-1.9855$
Therefore, $\mathrm{k}=\operatorname{Antilog}(-1.9855)$
$=1.034 \times 10^{-2} \mathrm{~s}^{-1}$
Question 30. The rate of a reaction quadruples when the temperature changes from $293 \mathrm{~K}$ to $313 \mathrm{~K}$. Calculate the energy of activation of the reaction assuming that it does not change with temperature.
Solution. Given: $\mathrm{k}_{2}=4 \mathrm{k}_{1}$
$\mathrm{T}_{1}=293 \mathrm{~K}$
$\mathrm{T}_{2}=313 \mathrm{~K}$
From the Arrhenius equation, we obtain
$\log \frac{\mathrm{k}_{2}}{\mathrm{k}_{1}}=\frac{\mathrm{E}_{\mathrm{a}}}{2.303 \mathrm{R}}\left(\frac{\mathrm{T}_{2}-\mathrm{T}_{1}}{\mathrm{~T}_{1} \mathrm{~T}_{2}}\right)$
Put all values from given data
We get $\log \frac{4 \mathrm{k}_{1}}{\mathrm{k}_{2}}=\frac{\mathrm{E}_{\mathrm{a}}}{2.303 \times 8.314}\left(\frac{313 \times 293}{293 \times 313}\right)$
$\Rightarrow 0.6021,=\frac{20 \times \mathrm{E}_{\mathrm{a}}}{2.303 \times 8.314 \times 293 \times 313}$
$\Rightarrow \mathrm{E}_{\mathrm{a}}=\frac{0.6021 \times 2.303 \times 8.314 \times 293 \times 313}{20}$
$=52863.33 \mathrm{~J} \mathrm{~mol}^{-1}$
$=52.86 \mathrm{~kJ} \mathrm{~mol}^{-1}$
Hence, the required energy of activation is $52.86 \mathrm{kJmol}^{-1}$.
Also Read,
Class 12 Chemistry Notes Free PDF.
Class 12 Chemistry Book Chapterwise Free PDF.
Class 12 Chemistry Exemplar Chapterwise Free PDF.
If you have any Confusion related to NCERT Solutions for Class 12 Chemistry Chapter 4 Chemical Kinetics PDF then feel free to ask in the comments section down below.
To watch Free Learning Videos on Class 12 Chemistry by Kota’s top IITan’s Faculties Install the eSaral App
