Hey, do you want to learn about Nuclear fission and fusion? if so. Then you are at the right place.
Nuclear fission
- The process of splitting of a heavy nucleus into two nuclei of comparable size and release of large energy is called fission.
${ }_{92} \mathrm{U}^{235}+{ }_{0} \mathrm{n}^{1} \longrightarrow{ }_{2}{ }_{2} \mathrm{U}^{236} \longrightarrow{ }_{56} \mathrm{Ba}^{141}+{ }_{36} \mathrm{Kr}^{92}+3{ }_{0} \mathrm{n}^{1}+$ energy $\mathrm{y}$- U235 nucleus captures a thermal neutron
- This forms a compound nucleus U236 in excited state
- The shape of nucleus is distorted and nucleus splits into two fragments emitting several neutrons.
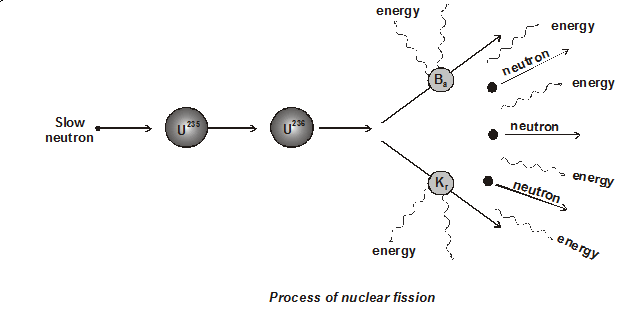
- Nuclear fission was discovered by Otto Hahn and Strassman.
- The energy of a thermal neutron ~ kT = 0.025 eV
- The binding energy per nucleon of products is greater than the reactants.
- The energy released in fission of Uranium is about 200 MeV. The fission energy released per nucleon is about 0.84 MeV
- The fission of U235 may take place by different routes but amount of energy released per fission is nearly equal

- The fission fragments are highly radioactive.
- Nuclear fission can be explained on basis of liquid drop model.
- The natural Uranium has following isotopes
${ }_{92} \mathrm{U}^{234}(0.006 \%) ; \quad{ }_{92} \mathrm{U}^{235}(0.72 \%) ; \quad{ }_{92} \mathrm{U}^{238}(99.27 \%)$ - 92U238 is not fissionable. This can be converted to plutonium which is fissionable by neutrons.
${ }_{92} \mathrm{U}^{238}+{ }_{0} \mathrm{n}^{1} \longrightarrow{ }_{93} \mathrm{~Np}^{239}+{ }_{-1} \mathrm{e}^{0}+\bar{v}$
${ }_{93} \mathrm{~Np}^{239} \longrightarrow{ }_{94} \mathrm{Pu}^{239}+{ }_{-1} \mathrm{e}^{0}+\bar{v}$ - The Uranium in which fraction of U235 is increased from 0.7% to 2.3% is called enriched Uranium.
- The neutrons emitted in fission are fast neutrons. Their energy is about 2MeV. On an average 2.5 neutrons are emitted per fission.
- Energy released per gm of Uranium
Energy released per gm of Uranium $=\frac{\text { Avogadro number }}{\text { mass number }} \times$ energy released per fission
$=\frac{6.023 \times 10^{23}}{235} \times 200=5.12 \times 10^{23} \mathrm{MeV}$
energy released by 1gm of U235 = 5.12 × 1023 MeV = 8.2 × 1010 J = 2.28 × 104 kWh = 2 × 1010 calorie
This energy is equivalent to
(i) energy obtained by burning 2560 kg of coal
(ii) energy obtained by burning 20 tonne of explosive TNT - The energy is released in form of kinetic energy of fission fragments, g-rays, heat, sound and light energy.
- The fission process can take place at normal pressure and temperature.
- Mass distribution in fission fragments
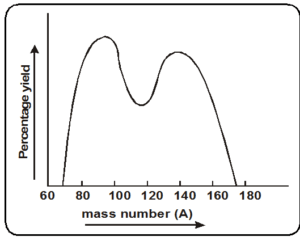
- The mass distribution of fission fragments is highly assymetric.
- The curve shows two peaks. at A = 95 and
A = 139. These represent the most probable mass numbers obtained in fission fragments. - The mass number of lighter fragment is between 85 to 104.
- The mass number of heavier fragment is between 130 to 149.
Nuclear fusion
- The process in which two or more lighter nuclei combine to form a heavy nucleus is known as nuclear fusion
$4_{1} \mathrm{H}^{1} \longrightarrow{ }_{2} \mathrm{He}^{4}+2_{+1} \mathrm{e}^{0}+2 \mathrm{v}+\mathrm{Q}$ - The binding energy per nucleon of product is greater than the reactants.
- The energy released per nucleon is large ~ 6.75 MeV.
- Fusion is possible at high pressure (~ 106 atom) and high temperature (~ 108 ºC)
- The proton-proton cycle happens at lower temperature as compared to carbon-nitrogen cycle.
- Nuclear fusion in possible at a place which has reactants in large quantity.
- Hydrogen bomb works on principle of nuclear fusion
- The explosion of a hydrogen bomb needs an explosion of atom bomb to generate required temperature.
- No harmful radiations are produced in fusion.
Difference Between Nuclear Fission and Nuclear Fusion
| S.no | Nuclear fission | Nuclear fusion |
| 1 | A heavy nucleus splits into two lighter nuclei | Two lighter nuclei join to form a heavy nucleus |
| 2 | Neutrons are required to start fission | Protons are usually required to start fusion |
| 3 | It takes place at normal pressure and temperature. |
It takes place at very high pressure and temperature. |
| 4 | The energy released per nucleon is small ~ 0.8 MeV |
The energy released per nucleon is large ~ 6 MeV |
| 5 | Fissionable material is expensive and not easily available |
Fusion material is cheap and abunduntly available |
| 6 | Energy released in fission per cycle is large ~200 MeV |
Energy released in fusion per cycle is small ~24.7 MeV |
For a better understanding of this chapter, please check the detailed notes of Nuclear Physics. If you want more Free Learning Videos and Study Material Then don't forget to download the eSaral App.
