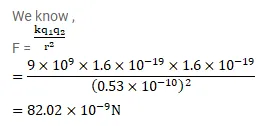Question:
A hydrogen atom contains one proton and one electron. It may be assumed that the electron revolves in a piece rod radius $0.53$ angstrom ( 1 angstrom $=10^{-10} \mathrm{~m}$ and is abbreviated as $\mathrm{A}$ ) with the proton at the centre. The hydrogen atom is said to be in the ground state in this case. Find the magnitude of the electric force between the proton and the electron of a hydrogen atom in its ground state.
Solution: