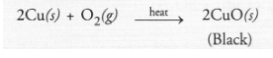Question:
A metal M does not liberate hydrogen from acids but reacts with oxygen to give a black coloured product. Identify M and black coloured product and
also explain the reaction of M with oxygen.
Solution:
The avilable information suggests that the metal M is copper (Cu). It does not liberate hydrogen since it is placed below hydrogen in the reactivity
series. Copper reacts with oxygen upon heating to form cupric oxide (CuO) which is black in colour.