Question:
Alkenes and carbonyl compounds both contain a π bond but alkenes show electrophilic addition reactions whereas carbonyl compounds show nucleophilic addition reactions. Explain.

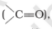
Solution:
Both the compounds carbon atom is attached to the electronegative atom oxygen. Thus the oxygen pulls more shared pair of electron towards them and a partial positive
charge will be acquired by carbon and a partial negative charge by oxygen. So carbonyl atom is attacked by a nucleophile.
