Question:
An element crystallises in a face-centred cubic (fcc) unit cell with cell edge a. The distance between the centres of two nearest octahedral voids in the crystal lattice is
Correct Option: 3,
Solution:
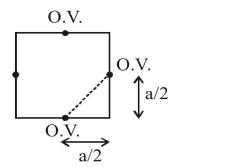
distance between nearest octahedral voids(O.V.)
$=\sqrt{\left(\frac{\mathrm{a}}{2}\right)^{2}+\left(\frac{\mathrm{a}}{2}\right)^{2}} \quad \Rightarrow=\frac{\mathrm{a}}{\sqrt{2}}$
