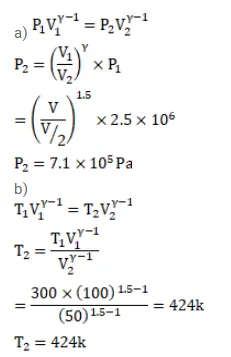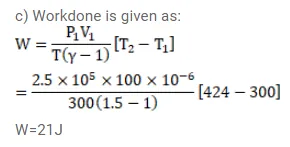Question:
An ideal gas at pressure $2.5 \times 10^{5} \mathrm{~Pa}$ and temperature $300 \mathrm{~K}$ occupies $100 \mathrm{cc}$. It is adiabatically compressed to half its original volume. Calculate
(a) the final pressure,
(b) the final temperature and
(c) the work done by the gas in the process. Take $\gamma=1.5$.
Solution: