Question:
Arrange the following labelled hydrogens in decreasing order of acidity:
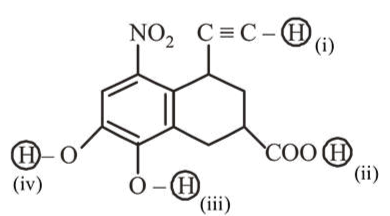
Correct Option: , 3
Solution:
Acidic strength $\propto$ Stability of conjugate base General order of acidic strength is
$\mathrm{R}-\mathrm{COOH}>\mathrm{Ph}-\mathrm{OH}>\mathrm{R}-\mathrm{C} \equiv \mathrm{CH}$
In between (iii) and (iv), (iii) is more acidic due to $-\mathrm{M}$ effect of $-\mathrm{NO}_{2}$.
Thus, decreasing order of acidity is (ii) $>$ (iii) $>$ (iv) $>$ (i).
