Question.
Calculate the concentration of nitric acid in moles per litre in a sample which has a density, $1.41 \mathrm{~g} \mathrm{~mL}^{-1}$ and the mass per cent of nitric acid in it being $69 \%$.
Calculate the concentration of nitric acid in moles per litre in a sample which has a density, $1.41 \mathrm{~g} \mathrm{~mL}^{-1}$ and the mass per cent of nitric acid in it being $69 \%$.
Solution:
Mass percent of nitric acid in the sample = 69 % [Given]
Thus, 100 g of nitric acid contains 69 g of nitric acid by mass.
Molar mass of nitric acid (HNO3)
$=\{1+14+3(16)\} \mathrm{g} \mathrm{mol}^{-1}$
= 1 + 14 + 48
$=63 \mathrm{~g} \mathrm{~mol}^{-1}$
Number of moles in $69 \mathrm{~g}$ of $\mathrm{HNO}_{3}$
$=\frac{69 \mathrm{~g}}{63 \mathrm{~g} \mathrm{~mol}^{-1}}$ $=1.095 \mathrm{~mol}$
Volume of 100g of nitric acid solution
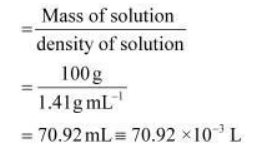
Concentration of nitric acid
$=\frac{1.095 \mathrm{~mole}}{70.92 \times 10^{-3} \mathrm{~L}}$ $=15.44 \mathrm{~mol} / \mathrm{L}$
com5Concentration of nitric acid = 15.44 mol/L
Mass percent of nitric acid in the sample = 69 % [Given]
Thus, 100 g of nitric acid contains 69 g of nitric acid by mass.
Molar mass of nitric acid (HNO3)
$=\{1+14+3(16)\} \mathrm{g} \mathrm{mol}^{-1}$
= 1 + 14 + 48
$=63 \mathrm{~g} \mathrm{~mol}^{-1}$
Number of moles in $69 \mathrm{~g}$ of $\mathrm{HNO}_{3}$
$=\frac{69 \mathrm{~g}}{63 \mathrm{~g} \mathrm{~mol}^{-1}}$ $=1.095 \mathrm{~mol}$
Volume of 100g of nitric acid solution
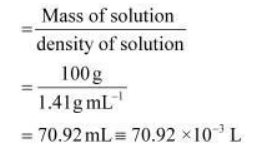
Concentration of nitric acid
$=\frac{1.095 \mathrm{~mole}}{70.92 \times 10^{-3} \mathrm{~L}}$ $=15.44 \mathrm{~mol} / \mathrm{L}$
com5Concentration of nitric acid = 15.44 mol/L
