Question:
Compare the structures of $\mathrm{H}_{2} \mathrm{O}$ and $\mathrm{H}_{2} \mathrm{O}_{2}$.
Solution:
In gaseous phase, water molecule has a bent form with a bond angle of $104.5^{\circ}$. The O-H bond length is $95.7$ pm. The structure can be shown as:
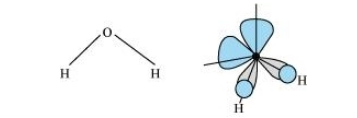
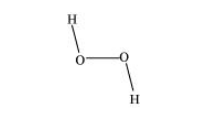
Hydrogen peroxide has a non-planar structure both in gas and solid phase. The dihedral angle in gas and solid phase is $111.5^{\circ}$ and $90.2^{\circ}$ respectively.
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
