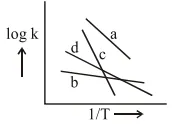
Question: Consider the following plots of rate constant versus $\frac{1}{T}$ for four different reactions. Which of the following orders is correct for the activation energies of these reactions?
$\mathrm{E}_{\mathrm{b}}>\mathrm{E}_{\mathrm{d}}>\mathrm{E}_{\mathrm{c}}>\mathrm{E}_{\mathrm{a}}$
$\mathrm{E}_{\mathrm{a}}>\mathrm{E}_{\mathrm{c}}>\mathrm{E}_{\mathrm{d}}>\mathrm{E}_{\mathrm{b}}$
$\mathrm{E}_{\mathrm{c}}>\mathrm{E}_{\mathrm{a}}>\mathrm{E}_{\mathrm{d}}>\mathrm{E}_{\mathrm{b}}$
$\mathrm{E}_{\mathrm{b}}>\mathrm{E}_{\mathrm{a}}>\mathrm{E}_{\mathrm{d}}>\mathrm{E}_{\mathrm{c}}$
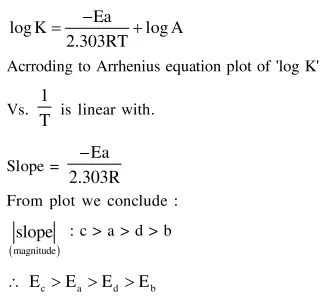
Correct Option: , 3
Solution: