Draw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation.
(a) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{OH}$
(b) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NO}_{2}$
(c) $\mathrm{CH}_{3} \mathrm{CH}=\mathrm{CH}-\mathrm{CHO}$
(d) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CHO}$
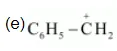
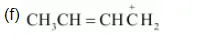
(a) The structure of $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{OH}$ is:
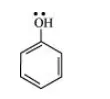
The resonating structures of phenol are represented as:
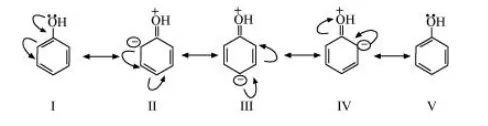
(b) The structure of $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NO}_{2}$ is:
The resonating structures of nitro benzene are represented as:
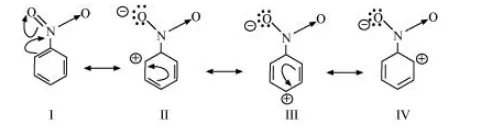
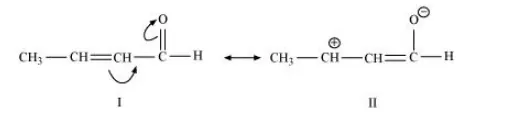
(c) $\mathrm{CH}_{3} \mathrm{CH}=\mathrm{CH}-\mathrm{CHO}$
The resonating structures of the given compound are represented as:
(d) The structure of $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CHO}$ is:
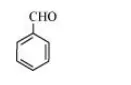
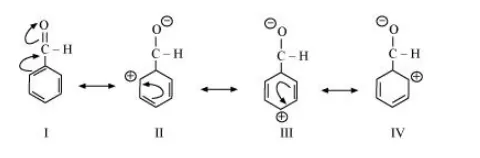
The resonating structures of benzaldehyde are represented as:
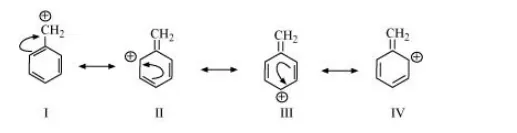
(e) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \oplus$
The resonating structures of the given compound are:
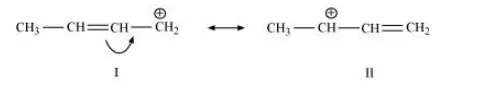
(f) $\mathrm{CH}_{3} \mathrm{CH}=\mathrm{CH} \mathrm{CH}_{2} \oplus$
The resonating structures of the given compound are: