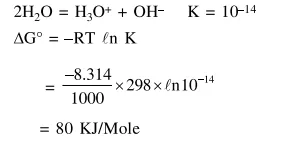
Question: For the equilibrium,
$2 \mathrm{H}_{2} \mathrm{O} \rightleftharpoons \mathrm{H}_{3} \mathrm{O}^{+}+\mathrm{OH}^{-}$, the value of $\Delta \mathrm{G}^{\circ}$ at 298
$\mathrm{K}$ is approximately :-
$-80 \mathrm{~kJ} \mathrm{~mol}^{-1}$
$-100 \mathrm{~kJ} \mathrm{~mol}^{-1}$
$100 \mathrm{~kJ} \mathrm{~mol}^{-1}$
$80 \mathrm{~kJ} \mathrm{~mol}^{-1}$
Correct Option: , 4
Solution: