Question:
Identify the most stable species in the following set of ions giving reasons:
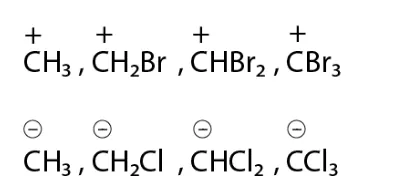
Solution:
(i) C+H3 will be more stable as the bromine atom destabilizes the positive charge on a carbon atom. Bromine atom is an electron-withdrawing group and has a lone pair of electrons.
(ii) CCl3 will be most stable because chlorine is more electron-withdrawing atom and the negative charge on carbon will be stabilized by the chlorine atom. As the number of chlorine atom attached to carbocation increases the stability also increases.
