Question:
If $\mathrm{AB}_{4}$ molecule is a polar molecule, a possible geometry of $\mathrm{AB}_{4}$ is :
Correct Option: 1
Solution:
(1) If $\mathrm{AB}_{4}$ molecule is a square pyramidal then it has one lone pair and their structure should be
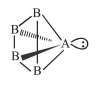
and it should be polar because dipole moment of lone pair of 'A' never be cancelled by others.
(2) If $\mathrm{AB}_{4}$ molecule is a tetrahedral then it has no lone pair and their structure should be
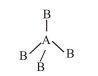
and it should be non polar due to perfect symmetry.
(3) If $\mathrm{AB}_{4}$ molecule is a square planar then
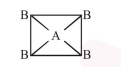
it should be non polar because vector sum of dipole moment is zero.
(4) If $\mathrm{AB}_{4}$ molecule is a rectangular planar then

it should be non polar because vector sum of dipole moment is zero.
