Question:
If $\mathrm{AB}_{4}$ molecule is a polar molecule, a possible geometry of $\mathrm{AB}_{4}$ is :
Correct Option: 1
Solution:
For $\mathrm{AB}_{4}$ compound possible geometry are
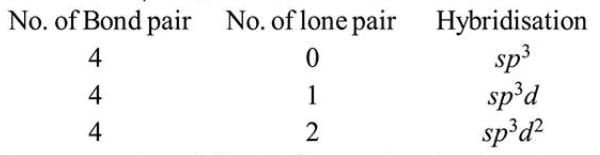
Structure with $s p^{3} d^{2}$ hybridisation is polar due to lone pair moment while in other possibilities molecules is non-polar. Square pyramidal can be polar due to lone pair moment as the bond pair moments will get cancelled out.
