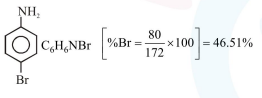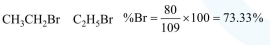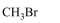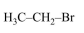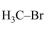Question:
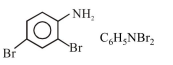
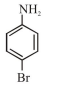
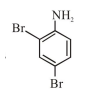
In Carius method of estimation of halogen, $0.172 \mathrm{~g}$ of an organic compound showed presence of $0.08 \mathrm{~g}$ of bromine. Which of these is the correct structure of the compound :
Correct Option: 1
Solution:
In Carius method
mass of organic compound $=0.172 \mathrm{gm}$
mass of Bromine $=0.08 \mathrm{gm}$
Hence $\%$ of Bromine $=\frac{0.08}{0.172} \times 100$
$=46.51 \%$