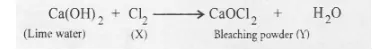
Question:
In one of the industrial processes used for the manufacture of sodium hydroxide, a gas ‘X’ is formed as by-product. The gas ‘X’ reacts with lime water to
give a compound ‘Y’ which is used as a bleaching agent in chemical industry. Identify ‘X’ and ‘Y’ giving the chemical equation of the reactions involved.
Solution:
Sodium hydroxide is manufactured by the electrolysis of a strong solution of sodium chloride (called brine). As a result, chlorine (X) is evolved at
anode while hydrogen at cathode. Chlorine reacts with lime water containing slaked lime to form bleaching power (Y)