Question:
In the ground state of atomic $\mathrm{Fe}(\mathrm{Z}=26)$, the spin-only magnetic moment is ____________ $\times 10^{-1} \mathrm{BM}$. (Round off to the Nearest Integer).
[Given : $\sqrt{3}=1.73, \sqrt{2}=1.41]$
Solution:
(49)
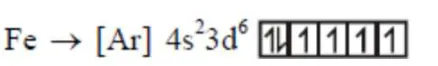
Number of unpaired $\mathrm{e}^{-}=4$
$\mu=\sqrt{4(4+2)} \mathrm{B} \cdot \mathrm{M}$
$\mu=\sqrt{24} \mathrm{~B} \cdot \mathrm{M}$
$\mu=4.89 \mathrm{~B} \cdot \mathrm{M}$
$\mu=48.9 \times 10^{-1} \mathrm{~B} \cdot \mathrm{M}$
Nearest integer value will be 49 .
