Question:
Lattice enthalpy and enthalpy of solution of $\mathrm{NaCl}$ are $788 \mathrm{~kJ} \mathrm{~mol}^{-1}$ and $4 \mathrm{~kJ} \mathrm{~mol}^{-1}$, respectively. The hydration enthalpy of $\mathrm{NaCl}$ is :
Correct Option: , 2
Solution:
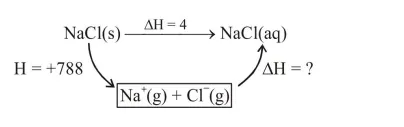
$4=788+\Delta \mathrm{H}$
$\Delta \mathrm{H}=-784 \mathrm{~kJ}$
