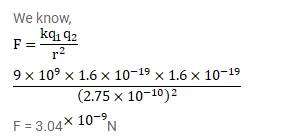Question:
$\mathrm{NaCl}$ molecule is bound due to the electric force between the sodium and the chlorine ions when one electron of sodium is transferred to chlorine. Taking the separation between the ions to be $2.75^{\times 10^{-8}} \mathrm{~cm}$, find the force of attraction between them. State the assumptions (if any) that you have made.
Solution: