Question:
Resonance structures of propenal are given below. Which of these resonating structures is more stable? Give a reason for your answer.
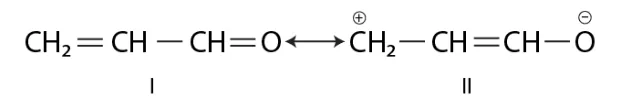
Solution:
The structure I will be more stable than structure II because all the atoms are having complete octet in structure I and the carbon atom having positive charge don’t have a complete octet in structure II.
