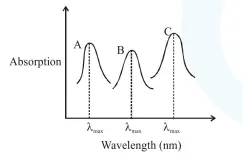
Simplified absorption spectra of three complexes ((i), (ii) and (iii)) of $\mathrm{M}^{\mathrm{nt}}$ ion are provided below; their $\lambda_{\max }$
values are marked as $\mathrm{A}, \mathrm{B}$ and $\mathrm{C}$ respectively. The correct match between the complexes and their $\lambda_{\max }$
values is :
(i) $\left[\mathrm{M}(\mathrm{NCS})_{6}\right]^{(-6+\mathrm{n})}$
(ii) $\left[\mathrm{MF}_{6}\right]^{(-6+\mathrm{n})}$
(iii) $\left[\mathrm{M}\left(\mathrm{NH}_{3}\right)_{6}\right]^{\mathrm{n}+}$
Correct Option: , 2
Strength of ligand $\mathrm{F}<\mathrm{NCS}^{-}<\mathrm{NH}_{3}$

As given in graph : $\mathrm{A}<\mathrm{B}<\mathrm{C} \quad\left(\lambda_{\max }\right)$
$\therefore$ Correct matching is $\mathrm{A}$-(iii), $\mathrm{B}$-(i), $\mathrm{C}$-(ii)
