Question: 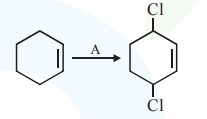
Identify the reagent(s) 'A' and condition(s) for the reaction :
$\mathrm{A}=\mathrm{HCl} ;$ Anhydrous $\mathrm{AlCl}_{3}$
$\mathrm{A}=\mathrm{HCl}, \mathrm{ZnCl}_{2}$
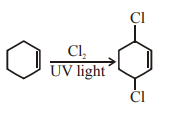
$\mathrm{A}=\mathrm{Cl}_{2} ; \mathrm{UV}$ light
$\mathrm{A}=\mathrm{Cl}_{2} ;$ dark, Anhydrous $\mathrm{AlCl}_{3}$
Correct Option: , 3
Solution:
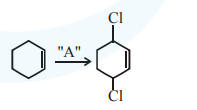
For substitution at allylic position in the given compound, the reagent used is $\mathrm{Cl}_{2} / \mathrm{uv}$ light.
The reaction is free radical halogenation.