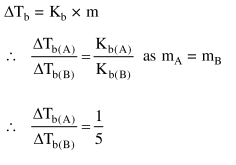Question:
$1 \mathrm{~g}$ of non-volatile non-electrolyte solute is dissolved in $100 \mathrm{~g}$ of two different solvents $A$ and B whose ebullioscopic constants are in the ratio of $1: 5$. The ratio of the elevation in their boiling
points, $\frac{\Delta \mathrm{T}_{\mathrm{b}}(\mathrm{A})}{\Delta \mathrm{T}_{\mathrm{b}}(\mathrm{B})}$, is :
Correct Option: , 3
Solution: