Question:
The bond order and the magnetic characteristics of $\mathrm{CN}^{-}$are:
Correct Option: , 2
Solution:
Total number of electrons in $\mathrm{CN}^{-}=6+7+1=14$
$\therefore$ Molecular orbital distribution
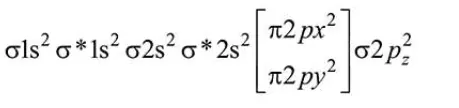
$\therefore$ Bond order $=\frac{10-4}{2}=3$
$\mathrm{CN}^{-}$is diamagnetic because all electrons are paired.
