Question:
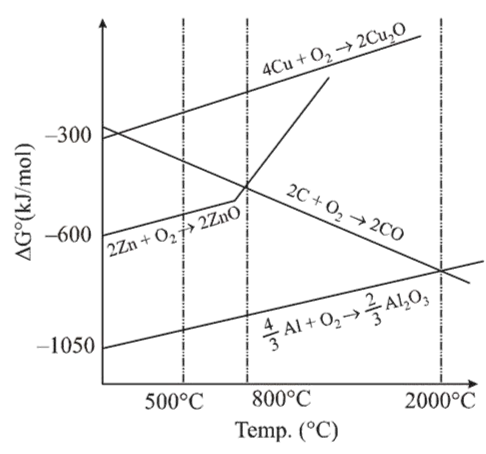
The correct statement regarding the given Ellingham diagram is:
Correct Option: 1
Solution:
In the given Ellingham diagram, the metal which has a lower value of $\Delta G^{\circ}$ (more negative) can reduce a metal oxide whose curve lies above it. So, Al can reduce $\mathrm{ZnO}$ at $1400^{\circ} \mathrm{C}$.
