Question:
The Crystal Field Stabilization Energy (CFSE) and magnetic moment (spin-only) of an octahedral aqua complex of a metal ion $\left(\mathrm{M}^{2+}\right)$ are $-0.8 \Delta_{0}$ and $3.87 \mathrm{BM}$, respectively. Identify $\left(\mathrm{M}^{2+}\right)$ :
Correct Option: , 4
Solution:
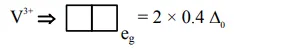
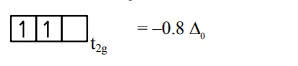
$=2$ unpaired $\mathrm{e}^{-}$
$\mu=2.89 \mathrm{Bm}$
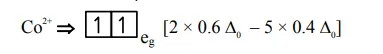
$=-0.8 \Delta_{0}$
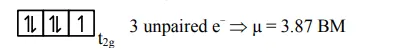
hence $\mathrm{d}^{7}$ configuration is of $\mathrm{Co}^{2+}$ Ans.
