Question:
The crystal Field stabilization Energy (CFSE) of $\left[\operatorname{CoF}_{3}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3}\right]\left(\Delta_{0}<\mathrm{P}\right)$ is :-
Correct Option: , 4
Solution:
$\left[\mathrm{CoF}_{3}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3}\right] \quad \Delta_{0}<\mathrm{P}$
Means all ligands behaves as weak field ligands
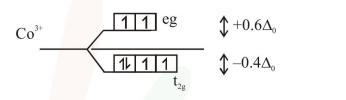
$=[-0.4 \times 4+0.6 \times 2] \Delta_{0}$
$=[-1.6+1.2] \Delta_{0}$
$=\left[-0.4 \Delta_{0}\right]$
