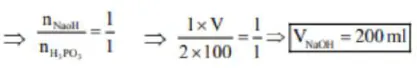The exact volumes of $1 \mathrm{MNaOH}$ solution required to neutralise $50 \mathrm{~mL}$ of $1 \mathrm{MH}_{3} \mathrm{PO}_{3}$ solution and $100 \mathrm{~mL}$ of $2 \mathrm{MH}_{3} \mathrm{PO}_{2}$ solution, respectively, are:
Correct Option: , 3
$\mathrm{H}_{3} \mathrm{PO}_{3}+2 \mathrm{NaOH} \rightarrow \mathrm{Na}_{2} \mathrm{HPO}_{3}+2 \mathrm{H}_{2} \mathrm{O}$
$50 \mathrm{ml}^{3} \quad 1 \mathrm{M}$
$1 \mathrm{M} \quad \mathrm{V}=$ ?
$\Rightarrow \frac{\mathrm{n}_{\mathrm{Nao} \mathrm{H}}}{\mathrm{n}_{\mathrm{H}_{1} \mathrm{PO}_{3}}}=\frac{2}{1}$
$\Rightarrow \frac{1 \times \mathrm{V}}{50 \times 1}=\frac{2}{1} \Rightarrow \mathrm{V}_{\mathrm{NaOH}}=100 \mathrm{ml}$
$\mathrm{H}_{3} \mathrm{PO}_{2}+2 \mathrm{NaOH} \rightarrow \mathrm{NaH}_{2} \mathrm{PO}_{3}+\mathrm{H}_{2} \mathrm{O}$
$100 \mathrm{ml}$
2M
$\mathrm{V}=?$