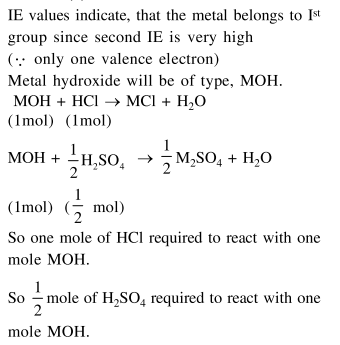
Question:
The first and second ionisation enthalpies of a metal are 496 and $4560 \mathrm{~kJ} \mathrm{~mol}^{-1}$, respectively. How many moles of $\mathrm{HCl}$ and $\mathrm{H}_{2} \mathrm{SO}_{4}$, respectively, will be needed to react completely with 1 mole of the metal hydroxide ?
Correct Option: 1
Solution: