Question:
The five successive ionization enthalpies of an element are $800,2427,3658,25024$ and 32824 $\mathrm{kJ} \mathrm{mol}^{-1}$. The number of valence electrons in the element is :
Correct Option: , 2
Solution:
Let suppose element $X \Rightarrow$
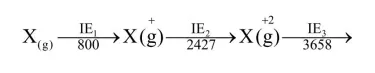
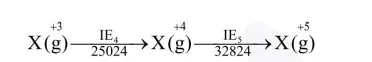
$\mathrm{X}^{+3}$ has stable inert gas configuration as there is high jump after $\mathrm{IE}_{3}$
So valence electrons are 3
