Question:
The Gibbs energy change (in J) for the given reaction at $\left[\mathrm{Cu}^{2+}\right]=\left[\mathrm{Sn}^{2+}\right]=1 \mathrm{M}$ and $298 \mathrm{~K}$ is:
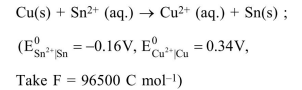
Official Ans. by NTA $(96500.00)$
Solution:
$\Delta \mathrm{G}=\Delta \mathrm{G}^{\circ}+\mathrm{RT} \ln \left[\frac{\mathrm{Sn}^{+2}}{\mathrm{Cu}^{+2}}\right]$
$=-2 \times 96500[(-0.16)-0.34]+\mathrm{RT} \ln \left(\frac{1}{1}\right)$
$=96500 \mathrm{~J}$
